Acids, Bases & SaltsPage
4
4
H+1 + OH-1 HOH
Acid + Base Water + Salt (double replacement)
HCl (aq) + NaOH (aq) HOH (l) + NaCl (aq)
H2SO4 (aq) + KOH (aq) 2 HOH (l) + K2SO4 (aq)
HBr (aq) + LiOH (aq)
H2CrO4 (aq) + NaOH (aq)
HNO3 (aq) + Ca(OH)2 (aq)
H3PO4 (aq) + Mg(OH)2 (aq)
جمعرات، 28 ربیع الثانی، 1438
Topic 10: ACIDS, BASES & SALTS
15
Slide 16
Topic 10: ACIDS, BASES & SALTS
16
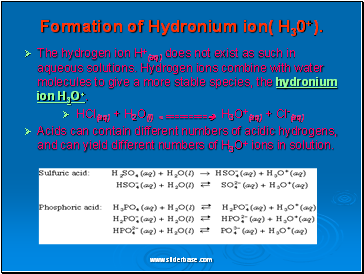
Formation of Hydronium ion( H30+).
The hydrogen ion H+(aq) does not exist as such in aqueous solutions. Hydrogen ions combine with water molecules to give a more stable species, the hydronium ion H3O+.
HCl(aq) + H2O(l) < ========= H3O+(aq) + Cl-(aq)
Acids can contain different numbers of acidic hydrogens, and can yield different numbers of H3O+ ions in solution.
جمعرات، 28 ربیع الثانی، 1438
Slide 17
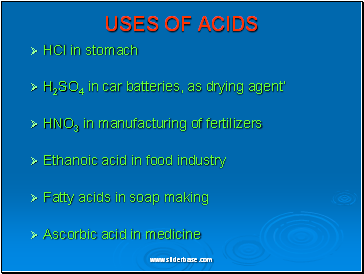
USES OF ACIDS
Topic 10: ACIDS, BASES & SALTS
17
HCl in stomach
H2SO4 in car batteries, as drying agent’
HNO3 in manufacturing of fertilizers
Ethanoic acid in food industry
Fatty acids in soap making
Ascorbic acid in medicine
جمعرات، 28 ربیع الثانی، 1438
Slide 18
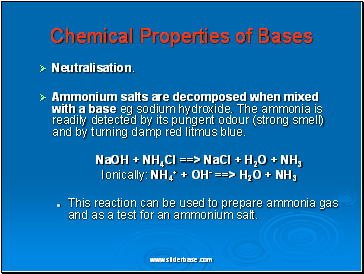
Chemical Properties of Bases
Topic 10: ACIDS, BASES & SALTS
18
Neutralisation.
Ammonium salts are decomposed when mixed with a base eg sodium hydroxide. The ammonia is readily detected by its pungent odour (strong smell) and by turning damp red litmus blue.
NaOH + NH4Cl ==> NaCl + H2O + NH3
Ionically: NH4+ + OH- ==> H2O + NH3
This reaction can be used to prepare ammonia gas and as a test for an ammonium salt.
جمعرات، 28 ربیع الثانی، 1438
Slide 19
Topic 10: ACIDS, BASES & SALTS
19
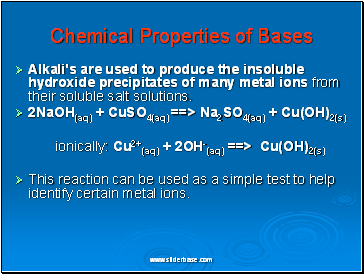
Chemical Properties of Bases
Alkali's are used to produce the insoluble hydroxide precipitates of many metal ions from their soluble salt solutions.
2NaOH(aq) + CuSO4(aq) ==> Na2SO4(aq) + Cu(OH)2(s)
ionically: Cu2+(aq) + 2OH-(aq) ==> Cu(OH)2(s)
This reaction can be used as a simple test to help identify certain metal ions.
جمعرات، 28 ربیع الثانی، 1438
Contents
- TERMS
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- USES OF ACIDS
- Chemical Properties of Bases
- TYPES OF OXIDES
- SALTS
- Methods of making Soluble Salts
- Making Insoluble Salts
- Types of Salts
- HYDRATED & ANHYDROUS SALTS
- Self Ionization of Water
- pH Graph
- IONIC EQUATIONS
Last added presentations
- The Effects of Radiation on Living Things
- Newton’s law of universal gravitation
- Waves & Sound
- Solar Energy
- Practical Applications of Solar Energy
- Radiation
- Simulation at NASA for the Space Radiation Effort
