Acids, Bases & SaltsPage
6
6
جمعرات، 28 ربیع الثانی، 1438
Slide 25
SALTS
Topic 10: ACIDS, BASES & SALTS
25
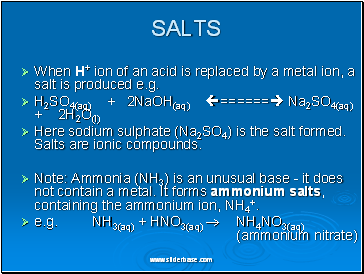
When H+ ion of an acid is replaced by a metal ion, a salt is produced e.g.
H2SO4(aq) + 2NaOH(aq) ====== Na2SO4(aq) + 2H2O(l)
Here sodium sulphate (Na2SO4) is the salt formed. Salts are ionic compounds.
Note: Ammonia (NH3) is an unusual base - it does not contain a metal. It forms ammonium salts, containing the ammonium ion, NH4+.
e.g. NH3(aq) + HNO3(aq) NH4NO3(aq) (ammonium nitrate)
جمعرات، 28 ربیع الثانی، 1438
Slide 26
Methods of making Soluble Salts
Topic 10: ACIDS, BASES & SALTS
26
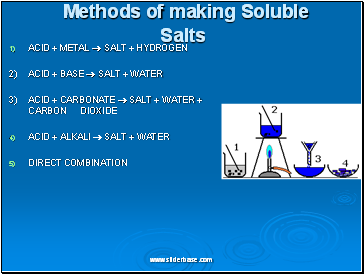
ACID + METAL SALT + HYDROGEN
2) ACID + BASE SALT + WATER
3) ACID + CARBONATE SALT + WATER + CARBON DIOXIDE
ACID + ALKALI SALT + WATER
DIRECT COMBINATION
جمعرات، 28 ربیع الثانی، 1438
Slide 27
Topic 10: ACIDS, BASES & SALTS
27
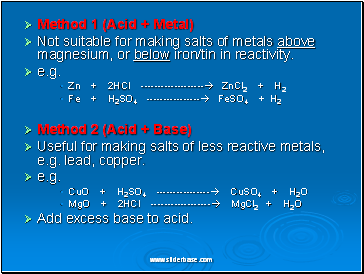
Method 1 (Acid + Metal)
Not suitable for making salts of metals above magnesium, or below iron/tin in reactivity.
e.g.
Zn + 2HCl ------------------- ZnCl2 + H2
Fe + H2SO4 ---------------- FeSO4 + H2
Method 2 (Acid + Base)
Useful for making salts of less reactive metals, e.g. lead, copper.
e.g.
CuO + H2SO4 ---------------- CuSO4 + H2O
MgO + 2HCl ------------------ MgCl2 + H2O
Add excess base to acid.
جمعرات، 28 ربیع الثانی، 1438
Slide 28
Topic 10: ACIDS, BASES & SALTS
28
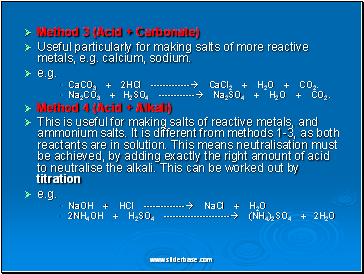
Method 3 (Acid + Carbonate)
Useful particularly for making salts of more reactive metals, e.g. calcium, sodium.
e.g.
CaCO3 + 2HCl ------------- CaCl2 + H2O + CO2.
Na2CO3 + H2SO4 ------------ Na2SO4 + H2O + CO2.
Method 4 (Acid + Alkali)
This is useful for making salts of reactive metals, and ammonium salts. It is different from methods 1-3, as both reactants are in solution. This means neutralisation must be achieved, by adding exactly the right amount of acid to neutralise the alkali. This can be worked out by titration
e.g.
NaOH + HCl -------------- NaCl + H2O
2NH4OH + H2SO4 ---------------------- (NH4)2SO4 + 2H2O
Contents
- TERMS
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- USES OF ACIDS
- Chemical Properties of Bases
- TYPES OF OXIDES
- SALTS
- Methods of making Soluble Salts
- Making Insoluble Salts
- Types of Salts
- HYDRATED & ANHYDROUS SALTS
- Self Ionization of Water
- pH Graph
- IONIC EQUATIONS
Last added presentations
- Sound
- Waves & Sound
- Newton’s laws of motion
- Upcoming Classes
- Friction
- Solar Thermal Energy
- Gravitation
