Acids, Bases & SaltsPage
9
9
Slide 40
Topic 10: ACIDS, BASES & SALTS
40
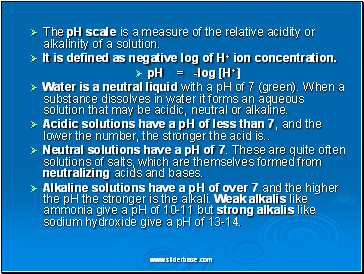
The pH scale is a measure of the relative acidity or alkalinity of a solution.
It is defined as negative log of H+ ion concentration.
pH = -log [H+]
Water is a neutral liquid with a pH of 7 (green). When a substance dissolves in water it forms an aqueous solution that may be acidic, neutral or alkaline.
Acidic solutions have a pH of less than 7, and the lower the number, the stronger the acid is
Neutral solutions have a pH of 7. These are quite often solutions of salts, which are themselves formed from neutralizing acids and bases.
Alkaline solutions have a pH of over 7 and the higher the pH the stronger is the alkali. Weak alkalis like ammonia give a pH of 10-11 but strong alkalis like sodium hydroxide give a pH of 13-14.
جمعرات، 28 ربیع الثانی، 1438
Slide 41
pH
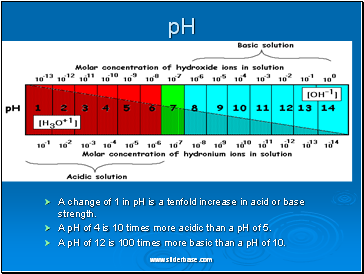
A change of 1 in pH is a tenfold increase in acid or base strength.
A pH of 4 is 10 times more acidic than a pH of 5.
A pH of 12 is 100 times more basic than a pH of 10.
جمعرات، 28 ربیع الثانی، 1438
Topic 10: ACIDS, BASES & SALTS
41
Slide 42
Topic 10: ACIDS, BASES & SALTS
42
جمعرات، 28 ربیع الثانی، 1438
Slide 43
Topic 10: ACIDS, BASES & SALTS
43
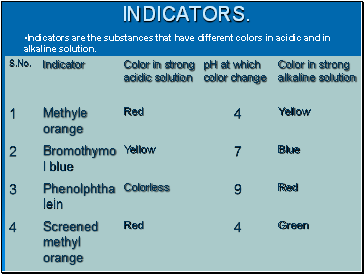
INDICATORS.
Indicators are the substances that have different colors in acidic and in alkaline solution.
جمعرات، 28 ربیع الثانی، 1438
Slide 44

Topic 10: ACIDS, BASES & SALTS
44
جمعرات، 28 ربیع الثانی، 1438
Slide 45
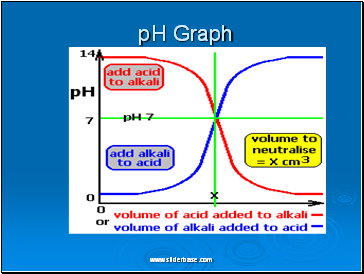
pH Graph
Topic 10: ACIDS, BASES & SALTS
45
جمعرات، 28 ربیع الثانی، 1438
Slide 46
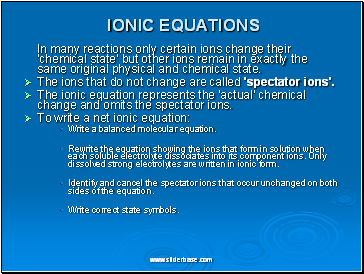
IONIC EQUATIONS
Topic 10: ACIDS, BASES & SALTS
46
In many reactions only certain ions change their 'chemical state' but other ions remain in exactly the same original physical and chemical state.
Contents
- TERMS
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- USES OF ACIDS
- Chemical Properties of Bases
- TYPES OF OXIDES
- SALTS
- Methods of making Soluble Salts
- Making Insoluble Salts
- Types of Salts
- HYDRATED & ANHYDROUS SALTS
- Self Ionization of Water
- pH Graph
- IONIC EQUATIONS
Last added presentations
- Newton's Laws
- Buoyancy
- Sound
- The Effects of Radiation on Living Things
- Newton’s law of universal gravitation
- Resource Acquisition and Transport in Vascular Plants
- Waves & Sound
