Bonding, Molecular Shape & StructurePage
4
4
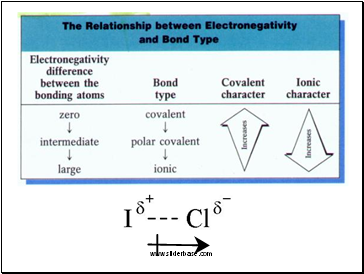
E.g. F-F (4.0 – 4.0 = 0) is non-polar covalent
H-F (4.0 – 2.1 = 1.9) is polar covalent
LiF (4.0 – 1.0 = 3.0) is ionic
+
-
Slide 23
Slide 24
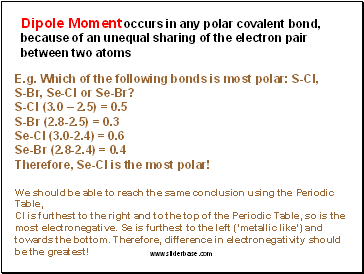
Dipole Moment occurs in any polar covalent bond, because of an unequal sharing of the electron pair between two atoms
E.g. Which of the following bonds is most polar: S-Cl,
S-Br, Se-Cl or Se-Br?
S-Cl (3.0 – 2.5) = 0.5
S-Br (2.8-2.5) = 0.3
Se-Cl (3.0-2.4) = 0.6
Se-Br (2.8-2.4) = 0.4
Therefore, Se-Cl is the most polar!
We should be able to reach the same conclusion using the Periodic Table,
Cl is furthest to the right and to the top of the Periodic Table, so is the most electronegative. Se is furthest to the left (‘metallic like’) and towards the bottom. Therefore, difference in electronegativity should be the greatest!
Slide 25
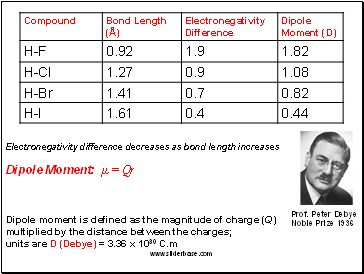
Electronegativity difference decreases as bond length increases
Dipole Moment: µ = Qr
Dipole moment is defined as the magnitude of charge (Q)
multiplied by the distance between the charges;
units are D (Debye) = 3.36 x 1030 C.m
Prof. Peter Debye
Noble Prize 1936
Slide 26
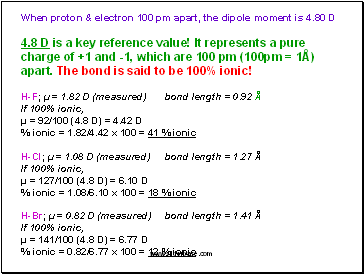
When proton & electron 100 pm apart, the dipole moment is 4.80 D
4.8 D is a key reference value! It represents a pure charge of +1 and -1, which are 100 pm (100pm = 1Å) apart. The bond is said to be 100% ionic!
H-F; µ = 1.82 D (measured) bond length = 0.92 Å
If 100% ionic,
µ = 92/100 (4.8 D) = 4.42 D
% ionic = 1.82/4.42 x 100 = 41 % ionic
H-Cl; µ = 1.08 D (measured) bond length = 1.27 Å
If 100% ionic,
µ = 127/100 (4.8 D) = 6.10 D
% ionic = 1.08/6.10 x 100 = 18 % ionic
H-Br; µ = 0.82 D (measured) bond length = 1.41 Å
If 100% ionic,
µ = 141/100 (4.8 D) = 6.77 D
% ionic = 0.82/6.77 x 100 = 12 % ionic
Slide 27
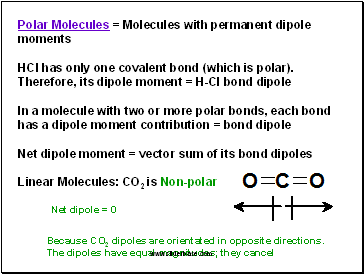
Polar Molecules = Molecules with permanent dipole moments
HCl has only one covalent bond (which is polar). Therefore, its dipole moment = H-Cl bond dipole
In a molecule with two or more polar bonds, each bond has a dipole moment contribution = bond dipole
Net dipole moment = vector sum of its bond dipoles
Linear Molecules: CO2 is Non-polar
Because CO2 dipoles are orientated in opposite directions.
The dipoles have equal magnitudes; they cancel
Net dipole = 0
Slide 28
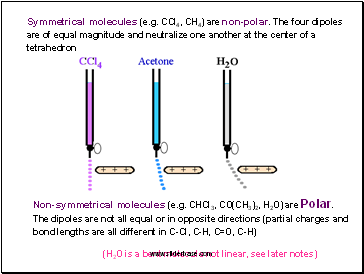
Symmetrical molecules (e.g. CCl4, CH4) are non-polar. The four dipoles are of equal magnitude and neutralize one another at the center of a tetrahedron
Contents
- Lewis Symbols
- Pauling scale of electronegativity;
- Electronegativity is dictated by
- Shapes of Molecules
- Trigonal Pyramidal
- Valence-Shell Electron-Pair Repulsion Theory (VSEPR)
- Valence Shell Electron-Pair Repulsion Theory (VSEPR)
- Molecules with Expanded Valence Shells
- Hydrogen Bonding & Water
- Dipole-dipole Attractive Forces
Last added presentations
- Sensory and Motor Mechanisms
- Space Radiation
- Sound
- Newton’s law of universal gravitation
- Magnetic field uses sound waves to ignite sun's ring of fire
- Friction
- Buoyancy