Chemical reactionsPage
3
3
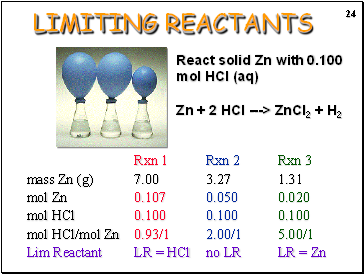
Rxn 2: Balloon inflates fully, no Zn left
* Right amount of each (HCl and Zn)
Rxn 3: Balloon does not inflate fully, no Zn left.
* Not enough Zn to use up 0.100 mol HCl
LIMITING REACTANTS
React solid Zn with 0.100 mol HCl (aq)
Zn + 2 HCl ---> ZnCl2 + H2
(See CD Screen 4.8)
Slide 24
Rxn 1 Rxn 2 Rxn 3
mass Zn (g) 7.00 3.27 1.31
mol Zn 0.107 0.050 0.020
mol HCl 0.100 0.100 0.100
mol HCl/mol Zn 0.93/1 2.00/1 5.00/1
Lim Reactant LR = HCl no LR LR = Zn
LIMITING REACTANTS
React solid Zn with 0.100 mol HCl (aq)
Zn + 2 HCl ---> ZnCl2 + H2
Slide 25
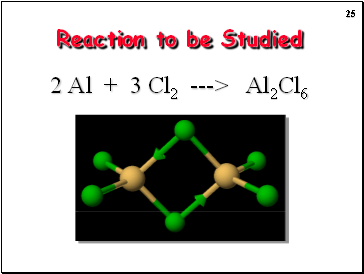
Reaction to be Studied
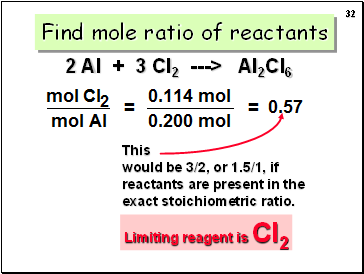
2 Al + 3 Cl2 ---> Al2Cl6
Slide 26
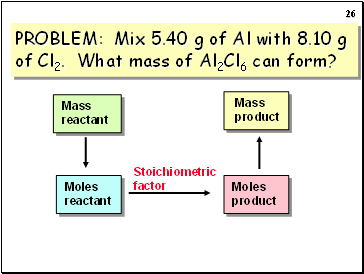
PROBLEM: Mix 5.40 g of Al with 8.10 g of Cl2. What mass of Al2Cl6 can form?
Stoichiometric
factor
Slide 27
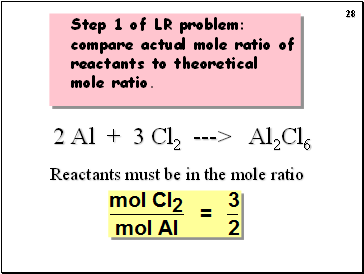
Step 1 of LR problem: compare actual mole ratio of reactants to theoretical mole ratio.
Slide 28
2 Al + 3 Cl2 ---> Al2Cl6
Reactants must be in the mole ratio
Step 1 of LR problem: compare actual mole ratio of reactants to theoretical mole ratio.
Slide 29
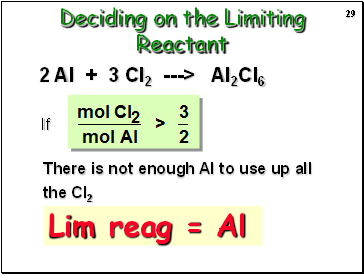
Deciding on the Limiting Reactant
If
There is not enough Al to use up all the Cl2
2 Al + 3 Cl2 ---> Al2Cl6
Lim reag = Al
Slide 30
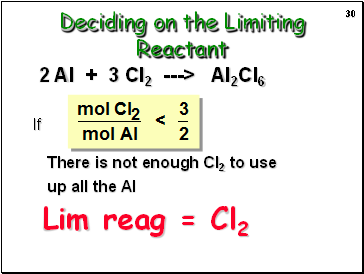
If
There is not enough Cl2 to use up all the Al
2 Al + 3 Cl2 ---> Al2Cl6
Lim reag = Cl2
Deciding on the Limiting Reactant
Slide 31
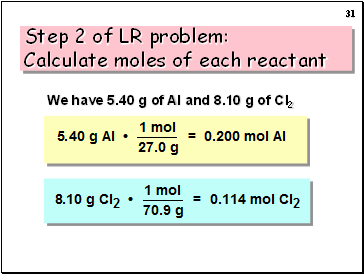
We have 5.40 g of Al and 8.10 g of Cl2
Step 2 of LR problem: Calculate moles of each reactant
Slide 32
Find mole ratio of reactants
Limiting reagent is Cl2
2 Al + 3 Cl2 ---> Al2Cl6
Slide 33
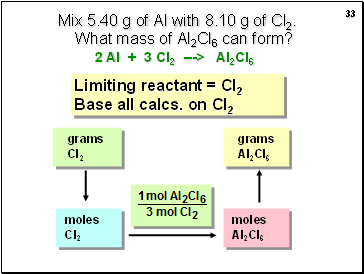
Mix 5.40 g of Al with 8.10 g of Cl2. What mass of Al2Cl6 can form?
Limiting reactant = Cl2
Base all calcs. on Cl2
2 Al + 3 Cl2 ---> Al2Cl6
Slide 34
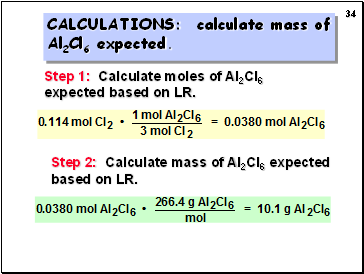
CALCULATIONS: calculate mass of
Al2Cl6 expected.
Step 1: Calculate moles of Al2Cl6 expected based on LR.
Step 2: Calculate mass of Al2Cl6 expected based on LR.
Slide 35
Contents
- Chemical Equations
- Balancing Equations
- Stoichiometry
- General plan for stoichiometry calculations
- Reactions Involving a Limiting reactant
- Limiting reactants
- Reaction to be Studied
- Using Stoichiometry to Determine a Formula
Last added presentations
- Space Radiation
- Ch 9 Nuclear Radiation
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Mechanics Lecture
- Direct heat utilization of geothermal energy
- Newton's laws of motion
- Sound