Hard-Soft Acid-Base TheoryPage
2
2
Slide 10
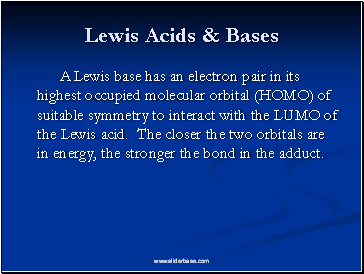
Lewis Acids & Bases
A Lewis base has an electron pair in its highest occupied molecular orbital (HOMO) of suitable symmetry to interact with the LUMO of the Lewis acid. The closer the two orbitals are in energy, the stronger the bond in the adduct.
Slide 11
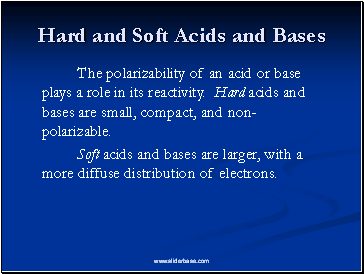
Hard and Soft Acids and Bases
The polarizability of an acid or base plays a role in its reactivity. Hard acids and bases are small, compact, and non-polarizable.
Soft acids and bases are larger, with a more diffuse distribution of electrons.
Slide 12
Hard and Soft Acids and Bases
In addition to their intrinsic strength,
Hard acids react preferentially with hard bases, and soft acids react preferentially with soft bases.
Slide 13
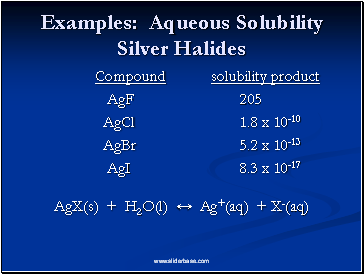
Examples: Aqueous Solubility Silver Halides
Compound solubility product
AgF 205
AgCl 1.8 x 10-10
AgBr 5.2 x 10-13
AgI 8.3 x 10-17
AgX(s) + H2O(l) ↔ Ag+(aq) + X-(aq)
Slide 14
Solubility of Lithium Halides
LiBr> LiCl> LiI> LiF
LiF should have a higher ∆solv than the other salts, yet it is the least soluble in water. This is due to the strong hard acid (Li+)/hard base (F-) interaction.
Slide 15
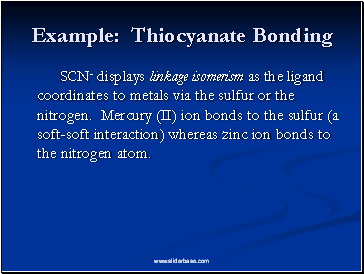
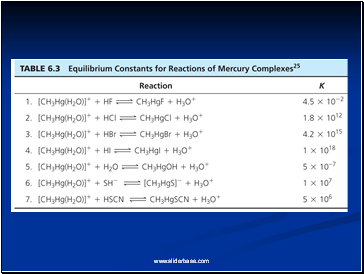
Example: Thiocyanate Bonding
SCN- displays linkage isomerism as the ligand coordinates to metals via the sulfur or the nitrogen. Mercury (II) ion bonds to the sulfur (a soft-soft interaction) whereas zinc ion bonds to the nitrogen atom.
Slide 16
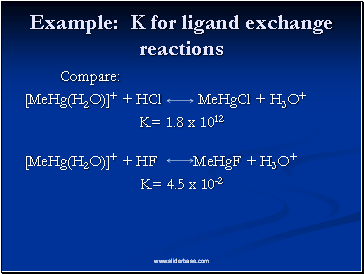
Example: K for ligand exchange reactions
Compare:
[MeHg(H2O)]+ + HCl MeHgCl + H3O+
K= 1.8 x 1012
[MeHg(H2O)]+ + HF MeHgF + H3O+
K= 4.5 x 10-2
Slide 17
Slide 18
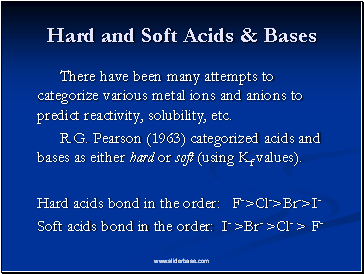
Hard and Soft Acids & Bases
There have been many attempts to categorize various metal ions and anions to predict reactivity, solubility, etc.
R.G. Pearson (1963) categorized acids and bases as either hard or soft (using Kf values).
Hard acids bond in the order: F->Cl->Br->I-
Soft acids bond in the order: I- >Br- >Cl- > F-
Slide 19
Hard and Soft Acids & Bases
Contents
- Definitions
- Other Solvents
- Lewis Acids & Bases
- Hard and Soft Acids and Bases
- Solubility of Lithium Halides
- Example: Thiocyanate Bonding
- Charge Density – Hard Acids
- Acids
- Bases
- Effect of Linkage Site
- Acid or Base Strength
- Applications of Hard/Soft Theory
- Evidence in Nature
Last added presentations
- Motion
- Sound
- Newton’s law of universal gravitation
- Buoyancy
- Newton’s Law of Gravity
- Newton’s laws of motion
- Space Radiation