Hard-Soft Acid-Base TheoryPage
3
3
Hard acids or bases are compact, with the electrons held fairly tightly by the nucleus. They are not very polarizable. F- is a hard base, and metal ions such as Li+, a hard acid.
Slide 20
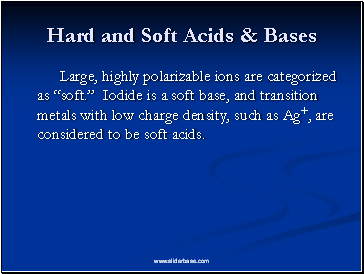
Hard and Soft Acids & Bases
Large, highly polarizable ions are categorized as “soft.” Iodide is a soft base, and transition metals with low charge density, such as Ag+, are considered to be soft acids.
Slide 21
Hard and Soft Acids & Bases
Hard acids tend to bind to hard bases.
Soft acids tend to bind to soft bases.
Slide 22
Problem
Predict the solubility (high or low) of silver fluoride, silver iodide, lithium fluoride and lithium iodide using the hard-soft acid/base approach. Identify each Lewis acid and Lewis base, and categorize each as hard or soft.
Slide 23
Charge Density – Hard Acids
Hard acids typically have a high charge density. They are often metal ions with a (higher) positive charge and small ionic size. Their d orbitals are often unavailable to engage in π bonding.
Slide 24
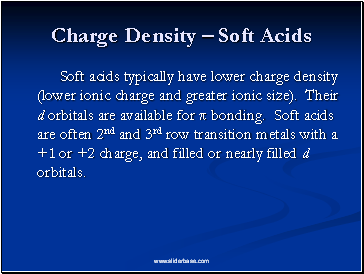
Charge Density – Soft Acids
Soft acids typically have lower charge density (lower ionic charge and greater ionic size). Their d orbitals are available for π bonding. Soft acids are often 2nd and 3rd row transition metals with a +1 or +2 charge, and filled or nearly filled d orbitals.
Slide 25
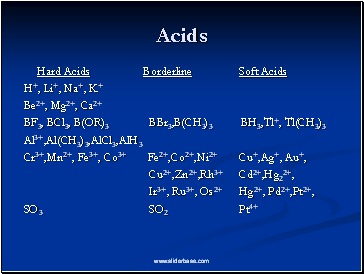
Acids
Hard Acids Borderline Soft Acids
H+, Li+, Na+, K+
Be2+, Mg2+, Ca2+
BF3, BCl3, B(OR)3 BBr3,B(CH3)3 BH3,Tl+, Tl(CH3)3
Al3+,Al(CH3)3,AlCl3,AlH3
Cr3+,Mn2+, Fe3+, Co3+ Fe2+,Co2+,Ni2+ Cu+,Ag+, Au+,
Cu2+,Zn2+,Rh3+ Cd2+,Hg22+,
Ir3+, Ru3+, Os2+ Hg2+, Pd2+,Pt2+,
SO3 SO2 Pt4+
Slide 26
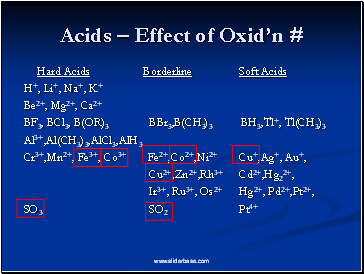
Acids – Effect of Oxid’n #
Hard Acids Borderline Soft Acids
H+, Li+, Na+, K+
Be2+, Mg2+, Ca2+
BF3, BCl3, B(OR)3 BBr3,B(CH3)3 BH3,Tl+, Tl(CH3)3
Al3+,Al(CH3)3,AlCl3,AlH3
Cr3+,Mn2+, Fe3+, Co3+ Fe2+,Co2+,Ni2+ Cu+,Ag+, Au+,
Cu2+,Zn2+,Rh3+ Cd2+,Hg22+,
Ir3+, Ru3+, Os2+ Hg2+, Pd2+,Pt2+,
SO3 SO2 Pt4+
Slide 27
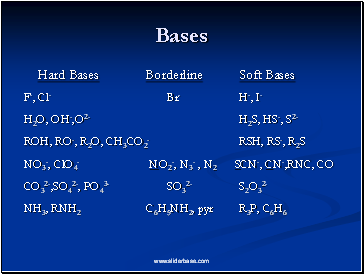
Bases
Hard Bases Borderline Soft Bases
F-, Cl- Br- H-, I-
H2O, OH-,O2- H2S, HS-, S2-
ROH, RO-, R2O, CH3CO2- RSH, RS-, R2S
NO3-, ClO4- NO2-, N3- , N2 SCN-, CN-,RNC, CO
CO32-,SO42-, PO43- SO32- S2O32-
Contents
- Definitions
- Other Solvents
- Lewis Acids & Bases
- Hard and Soft Acids and Bases
- Solubility of Lithium Halides
- Example: Thiocyanate Bonding
- Charge Density – Hard Acids
- Acids
- Bases
- Effect of Linkage Site
- Acid or Base Strength
- Applications of Hard/Soft Theory
- Evidence in Nature
Last added presentations
- Mechanics Lecture
- Static and Kinetic Friction
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Practical Applications of Solar Energy
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Direct heat utilization of geothermal energy
- Newton’s laws of motion