Hard-Soft Acid-Base TheoryPage
5
5
Slide 36
Other Considerations
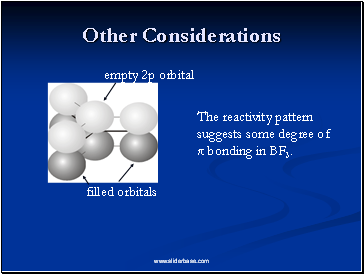
empty 2p orbital
filled orbitals
The reactivity pattern suggests some degree of π bonding in BF3.
Slide 37
Other Considerations
Steric factors can play a role. An example is the unfavorable reaction between :N(C6H5)3 and BCl3. The large phenyl groups interact with the chlorine atoms on boron to destabilize the product.
Slide 38
Applications of Hard/Soft Theory
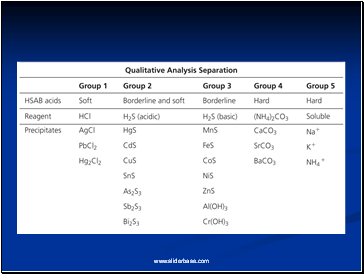
The Qual Scheme, a series of chemical reactions used to separate and identify the presence of dozens of metal ions, is based largely on the hard and soft properties of the metal ions.
The softer metals are precipitated out as chlorides or sulfides, with the harder ions formed as carbonates.
Slide 39
Slide 40
Evidence in Nature
In geochemistry, the elements in the earth’s crust are classified as lithophiles or chalcophiles.
The lithophile elements are typically found as silicates (bonded via the O atom): Li+, Mg2+, Ti3+, Al3+ and Cr2+,3+. These are hard Lewis acids.
Slide 41
Evidence in Nature
The chalcophile elements are typically found as sulfides or bonded to Se2- or Te2-. They include: Cd2+, Pb2+, Sb3+, and Bi3+. These are soft Lewis acids. Zinc ion, which is borderline, is typically found as a sulfide.
Contents
- Definitions
- Other Solvents
- Lewis Acids & Bases
- Hard and Soft Acids and Bases
- Solubility of Lithium Halides
- Example: Thiocyanate Bonding
- Charge Density – Hard Acids
- Acids
- Bases
- Effect of Linkage Site
- Acid or Base Strength
- Applications of Hard/Soft Theory
- Evidence in Nature
Last added presentations
- History of Modern Astronomy
- Solar Thermal Energy
- Newton's laws of motion
- Newton’s Laws of Motion
- Soil and Plant Nutrition
- The Effects of Radiation on Living Things
- Geophysical Concepts, Applications and Limitations