Polar Covalent Bonds Acids and BasesPage
4
4
Slide 23
23
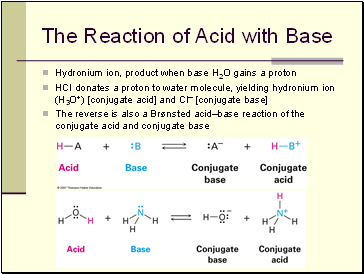
The Reaction of Acid with Base
Hydronium ion, product when base H2O gains a proton
HCl donates a proton to water molecule, yielding hydronium ion (H3O+) [conjugate acid] and Cl [conjugate base]
The reverse is also a Brønsted acid–base reaction of the conjugate acid and conjugate base
Slide 24
24
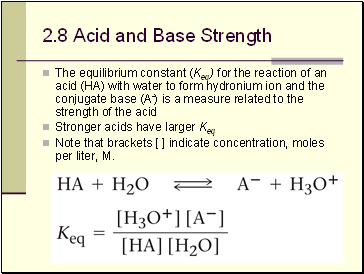
Acid and Base Strength
The equilibrium constant (Keq) for the reaction of an acid (HA) with water to form hydronium ion and the conjugate base (A-) is a measure related to the strength of the acid
Stronger acids have larger Keq
Note that brackets [ ] indicate concentration, moles per liter, M.
Slide 25
25
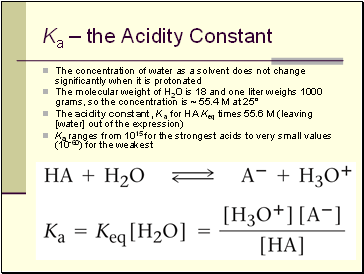
Ka – the Acidity Constant
The concentration of water as a solvent does not change significantly when it is protonated
The molecular weight of H2O is 18 and one liter weighs 1000 grams, so the concentration is ~ 55.4 M at 25°
The acidity constant, Ka for HA Keq times 55.6 M (leaving [water] out of the expression)
Ka ranges from 1015 for the strongest acids to very small values (10-60) for the weakest
Slide 26
26
Slide 27
27
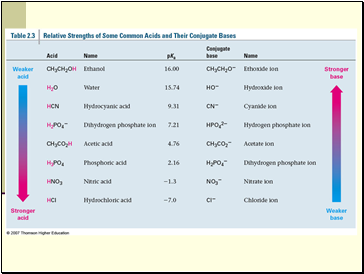
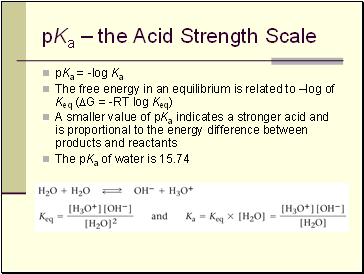
pKa – the Acid Strength Scale
pKa = -log Ka
The free energy in an equilibrium is related to –log of Keq (DG = -RT log Keq)
A smaller value of pKa indicates a stronger acid and is proportional to the energy difference between products and reactants
The pKa of water is 15.74
Slide 28
28
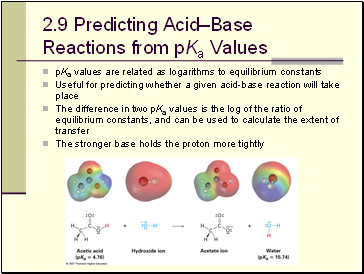
Predicting Acid–Base Reactions from pKa Values
pKa values are related as logariths to equilibrium constants
Useful for predicting whether a given acid-base reaction will take place
The difference in two pKa values is the log of the ratio of equilibrium constants, and can be used to calculate the extent of transfer
The stronger base holds the proton more tightly
Slide 29
29
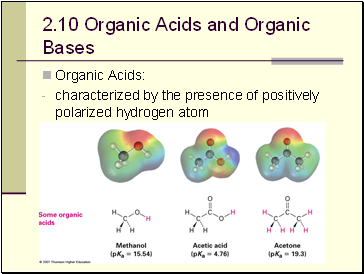
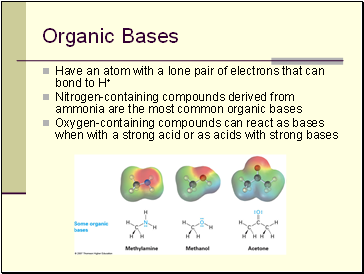
Organic Acids and Organic Bases
Organic Acids:
characterized by the presence of positively polarized hydrogen atom
Slide 30
30
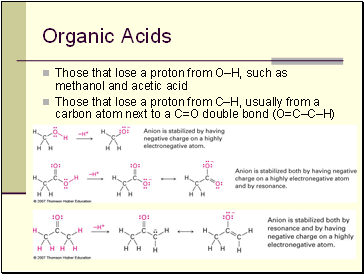
Organic Acids
Those that lose a proton from O–H, such as methanol and acetic acid
Those that lose a proton from C–H, usually from a carbon atom next to a C=O double bond (O=C–C–H)
Slide 31
Contents
- Why this chapter?
- Polar Covalent Bonds: Electronegativity
- Bond Polarity and Electronegativity
- Electrostatic Potential Maps
- Polar Covalent Bonds: Dipole Moments
- Formal Charges
- Resonance
- Rules for Resonance Forms
- Drawing Resonance Forms
- Pentanedione
- Acids and Bases: The Brønsted–Lowry Definition
- Acid and Base Strength
- Predicting Acid–Base Reactions from pKa Values
- Organic Acids and Organic Bases
- Acids and Bases: The Lewis Definition
- Molecular Models
- Noncovalent Interactions
Last added presentations
- Simulation at NASA for the Space Radiation Effort
- Madame Marie Curie
- The Effects of Radiation on Living Things
- Radiation Safety and Operations
- Buoyancy
- Motion
- Friction