Quantum Theory of the AtomPage
6
6
Slide 34
Copyright © Houghton Mifflin Company.All rights reserved.
Erwin Schrödinger
1926 -Nobel Prize in 1933
Found the probability of finding an electron in an atom, like flies to a candle.
Slide 35
Copyright © Houghton Mifflin Company.All rights reserved.
Erwin Schrödinger
1926 -Nobel Prize in 1933
Found the probability of finding an electron in an atom, like flies to a candle.
Slide 36
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–36
Quantum Mechanics
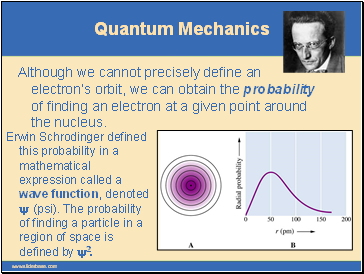
Although we cannot precisely define an electron’s orbit, we can obtain the probability of finding an electron at a given point around the nucleus.
Erwin Schrodinger defined this probability in a mathematical expression called a wave function, denoted y (psi). The probability of finding a particle in a region of space is defined by y2.
Slide 37
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–37
Quantum Numbers and Atomic Orbitals
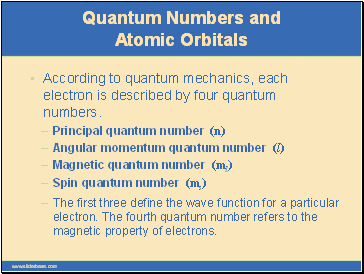
According to quantum mechanics, each electron is described by four quantum numbers.
The first three define the wave function for a particular electron. The fourth quantum number refers to the magnetic property of electrons.
Principal quantum number (n)
Angular momentum quantum number (l)
Magnetic quantum number (ml)
Spin quantum number (ms)
Slide 38
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–38
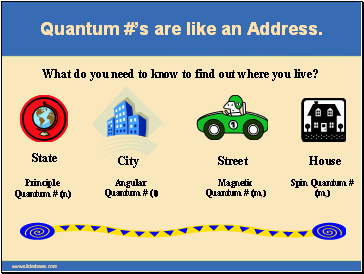
Quantum #’s are like an Address.
What do you need to know to find out where you live?
State
City
Street
House
Principle Quantum # (n)
Angular Quantum # (l)
Magnetic Quantum # (ml)
Spin Quantum # (ms)
Slide 39
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–39
The principal quantum number(n) represents the “shell number” in which an electron “resides.”
The smaller n is, the smaller the orbital.
The smaller n is, the lower the energy of the electron.
Quantum Numbers and Atomic Orbitals
Slide 40
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–40
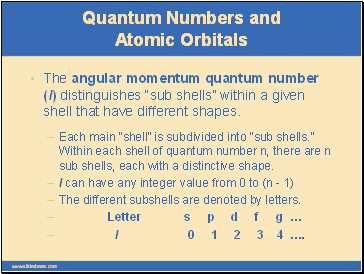
The angular momentum quantum number (l) distinguishes “sub shells” within a given shell that have different shapes.
Contents
- The Wave Nature of Light
- What is Quantized?
- Quantum Effects and Photons
- Radio Wave Energy
- A Problem to Consider
Last added presentations
- Newton’s Law of Gravity
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Static and Kinetic Friction
- Geophysical Concepts, Applications and Limitations
- Solar Energy
- Buoyancy
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal