Structure of the AtomPage
3
3
Electron crashes into the nucleus!?
Physics had reached a turning point in 1900 with Planck’s hypothesis of the quantum behavior of radiation.
Slide 15
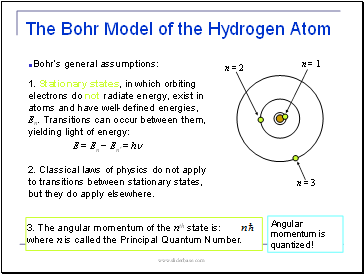
The Bohr Model of the Hydrogen Atom
Bohr’s general assumptions:
1. Stationary states, in which orbiting electrons do not radiate energy, exist in atoms and have well-defined energies, En. Transitions can occur between them, yielding light of energy:
E = En − En’ = hn
2. Classical laws of physics do not apply to transitions between stationary states, but they do apply elsewhere.
3. The angular momentum of the nth state is: where n is called the Principal Quantum Number.
Angular momentum is quantized!
Slide 16
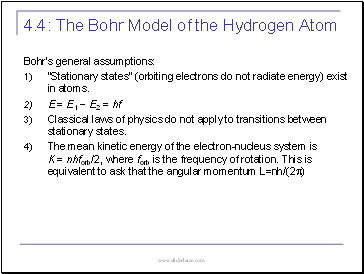
The Bohr Model of the Hydrogen Atom
4.4:
Bohr’s general assumptions:
“Stationary states” (orbiting electrons do not radiate energy) exist in atoms.
E = E1 − E2 = hf
Classical laws of physics do not apply to transitions between stationary states.
The mean kinetic energy of the electron-nucleus system is K = nhforb/2, where forb is the frequency of rotation. This is equivalent to ask that the angular momentum L=nh/(2p)
Slide 17
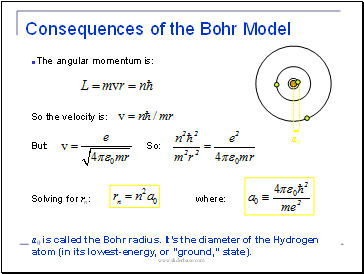
Consequences of the Bohr Model
The angular momentum is:
But:
So:
Solving for rn:
So the velocity is:
where:
a0 is called the Bohr radius. It’s the diameter of the Hydrogen atom (in its lowest-energy, or “ground,” state).
Slide 18
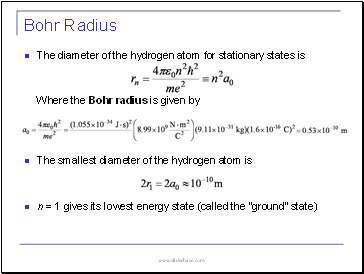
Bohr Radius
The diameter of the hydrogen atom for stationary states is
Where the Bohr radius is given by
The smallest diameter of the hydrogen atom is
n = 1 gives its lowest energy state (called the “ground” state)
Slide 19
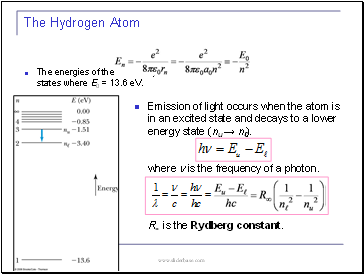
Emission of light occurs when the atom is in an excited state and decays to a lower energy state (nu → nℓ).
where n is the frequency of a photon.
R∞ is the Rydberg constant.
The Hydrogen Atom
The energies of the stationary states where E0 = 13.6 eV.
Slide 20
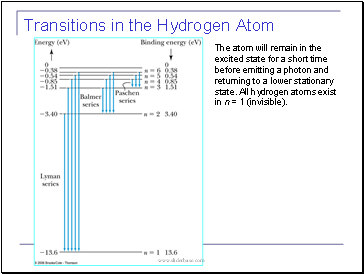
Transitions in the Hydrogen Atom
The atom will remain in the excited state for a short time before emitting a photon and returning to a lower stationary state. All hydrogen atoms exist in n = 1 (invisible).
Slide 21
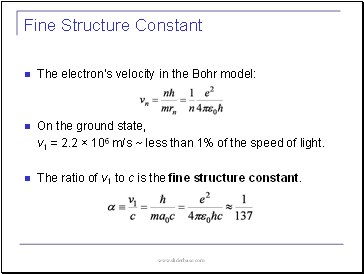
Fine Structure Constant
The electron’s velocity in the Bohr model:
On the ground state,
v1 = 2.2 × 106 m/s ~ less than 1% of the speed of light.
Contents
- Structure of the Atom
- Thomson’s Atomic Model
- Radius of an Atom
- Experiments of Geiger and Marsden
- Scattering from 1 electron:
- Multiple Scattering from Electrons
- Rutherford’s Atomic Model
- Rutherford Scattering
- The Classical Atomic Model
- The Bohr Model of the Hydrogen Atom
- Atomic Excitation by Electrons
Last added presentations
- Health Physics
- Newton’s Laws of Motion
- Ch 9 Nuclear Radiation
- Simulation at NASA for the Space Radiation Effort
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Solar Thermal Energy
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal