The Chemistry of Acids and BasesPage
4
4
pH = - log (1.0 x 10-11) = 11.00
Slide 34
The pH of rainwater collected in a certain region of the northeastern United States on a particular day was 4.82. What is the H+ ion concentration of the rainwater?
Slide 35
[OH-]
[H+]
pOH
pH
10-pOH
10-pH
-Log[H+]
-Log[OH-]
14 - pOH
14 - pH
1.0 x 10-14
[OH-]
1.0 x 10-14
[H+]
Slide 36
Calculating [H3O+], pH, [OH-], and pOH
Problem 1: A chemist dilutes concentrated hydrochloric acid to make two solutions: (a) 3.0 M and (b) 0.0024 M. Calculate the [H3O+], pH, [OH-], and pOH of the two solutions at 25°C.
Problem 2: What is the [H3O+], [OH-], and pOH of a solution with pH = 3.67? Is this an acid, base, or neutral?
Problem 3: Problem #2 with pH = 8.05?
Slide 37
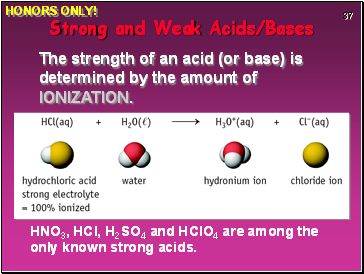
HNO3, HCl, H2SO4 and HClO4 are among the only known strong acids.
Strong and Weak Acids/Bases
The strength of an acid (or base) is determined by the amount of IONIZATION.
HONORS ONLY!
Slide 38
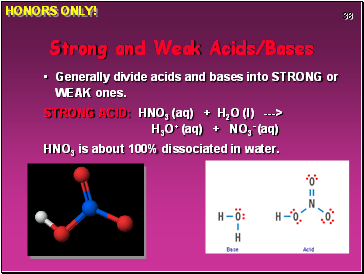
Strong and Weak Acids/Bases
Generally divide acids and bases into STRONG or WEAK ones.
STRONG ACID: HNO3 (aq) + H2O (l) ---> H3O+ (aq) + NO3- (aq)
HNO3 is about 100% dissociated in water.
HONORS ONLY!
Slide 39
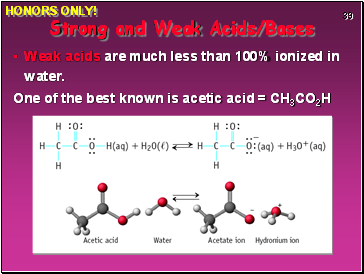
Weak acids are much less than 100% ionized in water.
One of the best known is acetic acid = CH3CO2H
Strong and Weak Acids/Bases
HONORS ONLY!
Slide 40
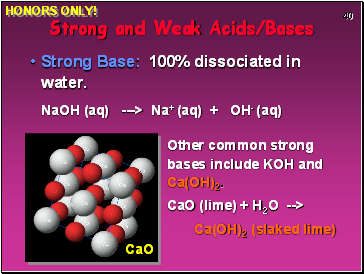
Strong Base: 100% dissociated in water.
NaOH (aq) ---> Na+ (aq) + OH- (aq)
Strong and Weak Acids/Bases
Other common strong bases include KOH and Ca(OH)2.
CaO (lime) + H2O -->
Ca(OH)2 (slaked lime)
HONORS ONLY!
Slide 41
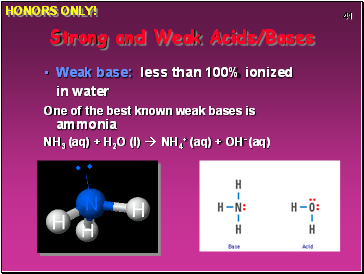
Weak base: less than 100% ionized in water
One of the best known weak bases is ammonia
NH3 (aq) + H2O (l) NH4+ (aq) + OH- (aq)
Strong and Weak Acids/Bases
HONORS ONLY!
Slide 42
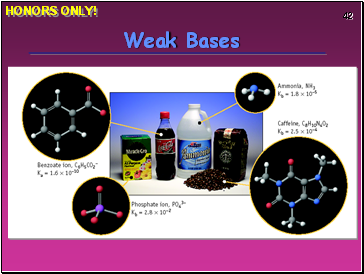
Weak Bases
HONORS ONLY!
Slide 43
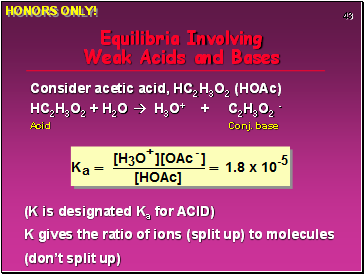
Equilibria Involving Weak Acids and Bases
Consider acetic acid, HC2H3O2 (HOAc)
HC2H3O2 + H2O H3O+ + C2H3O2 -
Acid Conj. base
(K is designated Ka for ACID)
Contents
- Acid and Bases
- Some Properties of Acids
- Acid Nomenclature Review
- Some Properties of Bases
- Some Common Bases
- Acid/Base definitions
- Lewis Acids & Bases
- Titration
Last added presentations
- Heat-Energy on the Move
- Soil and Plant Nutrition
- Motion
- Waves & Sound
- Direct heat utilization of geothermal energy
- Upcoming Classes
- Gravitation


![[OH-] [OH-]](images/referats/1143/image035.png)
![Calculating [H3O+], pH, [OH-], and pOH Calculating [H3O+], pH, [OH-], and pOH](images/referats/1143/image036.png)