The Chemistry of Acids and BasesPage
6
6
Equilibria Involving A Weak Acid
Calculate the pH of a 0.0010 M solution of formic acid, HCO2H.
HCO2H + H2O HCO2- + H3O+
Ka = 1.8 x 10-4
Approximate solution
[H3O+] = 4.2 x 10-4 M, pH = 3.37
Exact Solution
[H3O+] = [HCO2-] = 3.4 x 10-4 M
[HCO2H] = 0.0010 - 3.4 x 10-4 = 0.0007 M
pH = 3.47
HONORS ONLY!
Slide 54
Equilibria Involving A Weak Base
You have 0.010 M NH3. Calc. the pH.
NH3 + H2O NH4+ + OH-
Kb = 1.8 x 10-5
Step 1. Define equilibrium concs. in ICE table
[NH3] [NH4+] [OH-]
initial
change
equilib
0.010 0 0
-x +x +x
0.010 - x x x
HONORS ONLY!
Slide 55
Equilibria Involving A Weak Base
You have 0.010 M NH3. Calc. the pH.
NH3 + H2O NH4+ + OH-
Kb = 1.8 x 10-5
Step 1. Define equilibrium concs. in ICE table
[NH3] [NH4+] [OH-]
initial
change
equilib
0.010 0 0
-x +x +x
0.010 - x x x
HONORS ONLY!
Slide 56
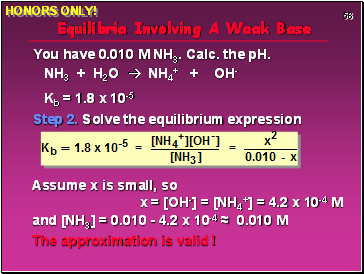
Equilibria Involving A Weak Base
You have 0.010 M NH3. Calc. the pH.
NH3 + H2O NH4+ + OH-
Kb = 1.8 x 10-5
Step 2. Solve the equilibrium expression
Assume x is small, so
x = [OH-] = [NH4+] = 4.2 x 10-4 M
and [NH3] = 0.010 - 4.2 x 10-4 ≈ 0.010 M
The approximation is valid !
HONORS ONLY!
Slide 57
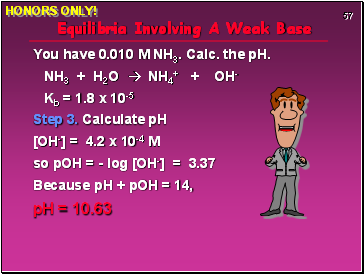
Equilibria Involving A Weak Base
You have 0.010 M NH3. Calc. the pH.
NH3 + H2O NH4+ + OH-
Kb = 1.8 x 10-5
Step 3. Calculate pH
[OH-] = 4.2 x 10-4 M
so pOH = - log [OH-] = 3.37
Because pH + pOH = 14,
pH = 10.63
HONORS ONLY!
Slide 58
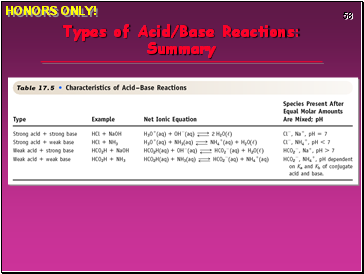
Types of Acid/Base Reactions: Summary
HONORS ONLY!
Slide 59
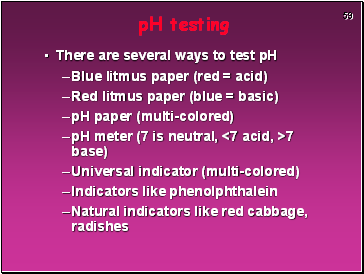
pH testing
There are several ways to test pH
Blue litmus paper (red = acid)
Red litmus paper (blue = basic)
pH paper (multi-colored)
pH meter (7 is neutral, <7 acid, >7 base)
Universal indicator (multi-colored)
Indicators like phenolphthalein
Natural indicators like red cabbage, radishes
Slide 60
Paper testing
Paper tests like litmus paper and pH paper
Put a stirring rod into the solution and stir.
Take the stirring rod out, and place a drop of the solution from the end of the stirring rod onto a piece of the paper
Read and record the color change. Note what the color indicates.
Contents
- Acid and Bases
- Some Properties of Acids
- Acid Nomenclature Review
- Some Properties of Bases
- Some Common Bases
- Acid/Base definitions
- Lewis Acids & Bases
- Titration
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Soil and Plant Nutrition
- Sound
- Radiation
- Sound
- Radioactivity and Nuclear Reactions
- Buoyancy