The pH ScalePage
3
3
Slide 21
LecturePLUS Timberlake
21
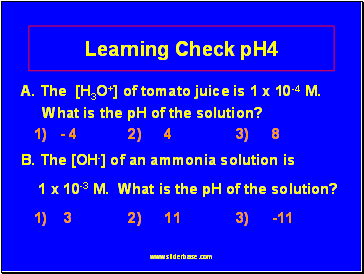
Learning Check pH4
A. The [H3O+] of tomato juice is 1 x 10-4 M.
What is the pH of the solution?
1) - 4 2) 4 3) 8
B. The [OH-] of an ammonia solution is
1 x 10-3 M. What is the pH of the solution?
1) 3 2) 11 3) -11
Slide 22
LecturePLUS Timberlake
22
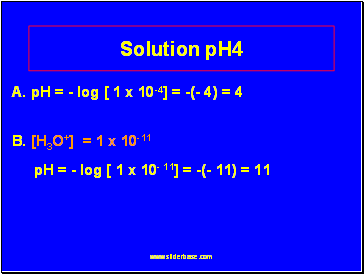
Solution pH4
A. pH = - log [ 1 x 10-4] = -(- 4) = 4
B. [H3O+] = 1 x 10-11
pH = - log [ 1 x 10- 11] = -(- 11) = 11
Slide 23
LecturePLUS Timberlake
23
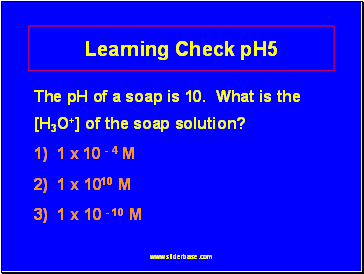
Learning Check pH5
The pH of a soap is 10. What is the [H3O+] of the soap solution?
1) 1 x 10 - 4 M
2) 1 x 1010 M
3) 1 x 10 - 10 M
Slide 24
LecturePLUS Timberlake
24
Solution pH5
The pH of a soap is 10. What is the [H3O+] of the soap solution?
[H3O+] = 1 x 10-pH M
= 1 x 10-10 M
Slide 25
LecturePLUS Timberlake
25
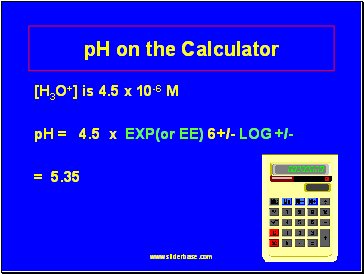
pH on the Calculator
[H3O+] is 4.5 x 10-6 M
pH = 4.5 x EXP(or EE) 6+/- LOG +/-
= 5.35
Slide 26
LecturePLUS Timberlake
26
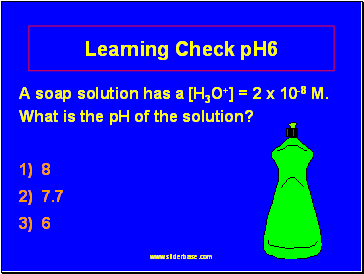
Learning Check pH6
A soap solution has a [H3O+] = 2 x 10-8 M. What is the pH of the solution?
1) 8
2) 7.7
3) 6
Slide 27
LecturePLUS Timberlake
27
Solution pH6
A soap solution has a [H3O+] = 2.0 x 10-8 M. What is the pH of the solution?
B) 2.0 EE 8 +/- LOG +/- = 7.7
Slide 28
LecturePLUS Timberlake
28
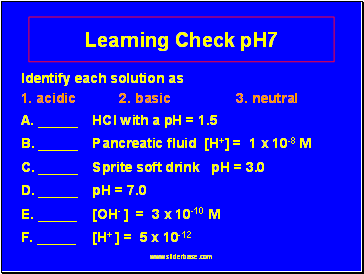
Learning Check pH7
Identify each solution as
1. acidic 2. basic 3. neutral
A. _ HCl with a pH = 1.5
B. _ Pancreatic fluid [H+] = 1 x 10-8 M
C. _ Sprite soft drink pH = 3.0
D. _ pH = 7.0
E. _ [OH- ] = 3 x 10-10 M
F. _ [H+ ] = 5 x 10-12
Slide 29
LecturePLUS Timberlake
29
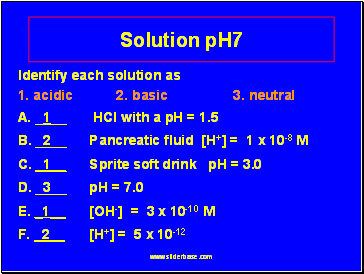
Solution pH7
Identify each solution as
1. acidic 2. basic 3. neutral
A. _1 HCl with a pH = 1.5
B. _2 Pancreatic fluid [H+] = 1 x 10-8 M
C. _1 Sprite soft drink pH = 3.0
D. _3 pH = 7.0
E. _1 [OH-] = 3 x 10-10 M
F. _2 [H+] = 5 x 10-12
Slide 30
Acid Rain
LecturePLUS Timberlake
30
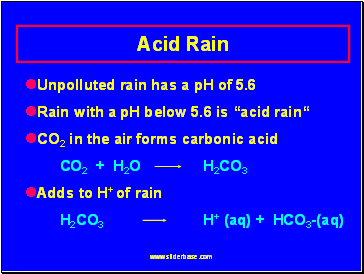
Unpolluted rain has a pH of 5.6
Contents
- Ionization of Water
- Pure Water is Neutral
- Ion Product of Water Kw
- Acids
- Bases
- Using the Calculator
- Acid Rain
- Sources of Acid Rain
- Effects of Acid Rain
Last added presentations
- Motion
- Heat-Energy on the Move
- Health Physics
- Radioactivity and Nuclear Reactions
- Newton's laws of motion
- Static and Kinetic Friction
- Gravitation