Making ElectricityPage
2
2
Slide 9
Magnesium is higher in the electrochemical series than copper.
Magnesium gives electrons to the copper ions.
The copper ions gaining these electrons form copper atoms (brown solid).
The magnesium atoms lose electrons to form colourless ions which dissolve in the solution.
Slide 10
The solution was blue due to the copper(II) ions.
As the copper ions are being changed to copper atoms, the blue colour fades.
The copper ions have been displaced from the solution as copper atoms.
A displacement reaction will occur when a metal is placed in a solution of metal ions, if the metal is higher in the electrochemical series than the metal ions.
Slide 11
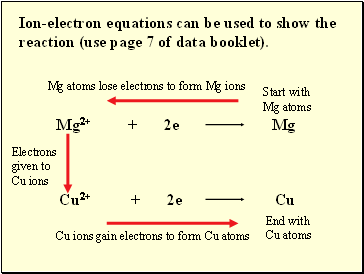
Ion-electron equations can be used to show the reaction (use page 7 of data booklet).
Start with
Mg atoms
End with
Cu atoms
Mg atoms lose electrons to form Mg ions
Electrons given to Cu ions
Cu ions gain electrons to form Cu atoms
Slide 12
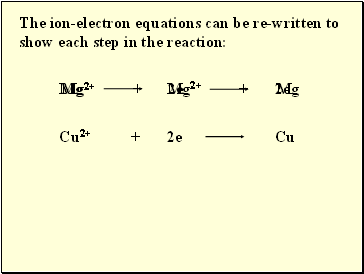
The ion-electron equations can be re-written to show each step in the reaction:
Slide 13
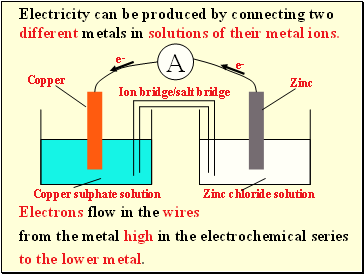
Electricity can be produced by connecting two different metals in solutions of their metal ions.
to the lower metal.
Electrons flow in the wires
from the metal high in the electrochemical series
Ion bridge/salt bridge
Slide 14
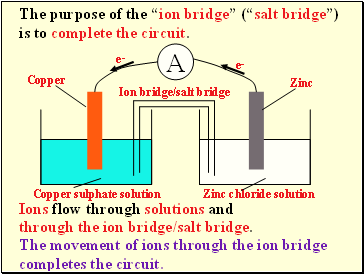
The purpose of the “ion bridge” (“salt bridge”) is to complete the circuit.
through the ion bridge/salt bridge.
Ions flow through solutions and
Ion bridge/salt bridge
The movement of ions through the ion bridge completes the circuit.
Slide 15
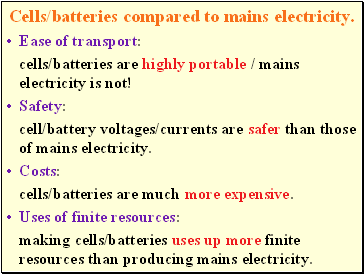
Cells/batteries compared to mains electricity.
Ease of transport:
cells/batteries are highly portable / mains electricity is not!
Safety:
cell/battery voltages/currents are safer than those of mains electricity.
Costs:
cells/batteries are much more expensive.
Uses of finite resources:
making cells/batteries uses up more finite resources than producing mains electricity.
Slide 16
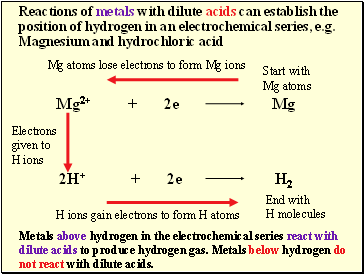
Reactions of metals with dilute acids can establish the position of hydrogen in an electrochemical series, e.g. Magnesium and hydrochloric acid
Start with
Mg atoms
End with
H molecules
Mg atoms lose electrons to form Mg ions
Contents
- Making Electricity
- Dry Cells
- Displacement reactions.
- Cells/batteries compared to mains electricity.
- Oxidation and Reduction
Last added presentations
- History of Modern Astronomy
- Radiation
- Simulation at NASA for the Space Radiation Effort
- Heat-Energy on the Move
- Newton's Laws
- Waves & Sound
- Newton's laws of motion