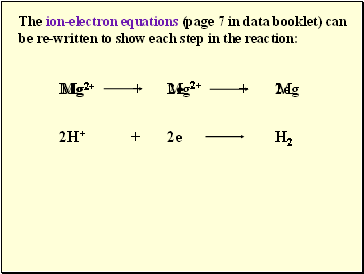Making ElectricityPage
3
3
Electrons given to H ions
H ions gain electrons to form H atoms
Metals above hydrogen in the electrochemical series react with dilute acids to produce hydrogen gas. Metals below hydrogen do not react with dilute acids.
Slide 17
The ion-electron equations (page 7 in data booklet) can be re-written to show each step in the reaction:
Slide 18
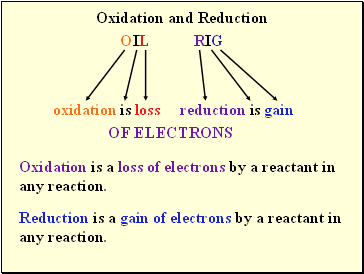
Oxidation and Reduction
OIL RIG
oxidation is loss reduction is gain
OF ELECTRONS
Oxidation is a loss of electrons by a reactant in any reaction.
Reduction is a gain of electrons by a reactant in any reaction.
Slide 19
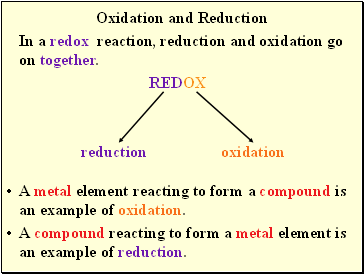
Oxidation and Reduction
REDOX
reduction oxidation
In a redox reaction, reduction and oxidation go on together.
A metal element reacting to form a compound is an example of oxidation.
A compound reacting to form a metal element is an example of reduction.
Slide 20
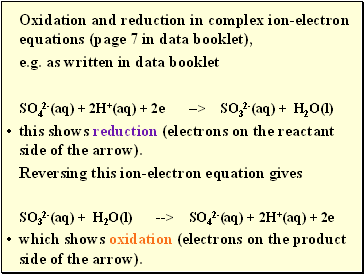
Oxidation and reduction in complex ion-electron equations (page 7 in data booklet),
e.g. as written in data booklet
SO42-(aq) + 2H+(aq) + 2e --> SO32-(aq) + H2O(l)
this shows reduction (electrons on the reactant side of the arrow).
Reversing this ion-electron equation gives
SO32-(aq) + H2O(l) --> SO42-(aq) + 2H+(aq) + 2e
which shows oxidation (electrons on the product side of the arrow).
Contents
- Making Electricity
- Dry Cells
- Displacement reactions.
- Cells/batteries compared to mains electricity.
- Oxidation and Reduction
Last added presentations
- Upcoming Classes
- Buoyancy
- Newton's Laws
- Space Radiation
- Waves & Sound
- Radioactivity and Nuclear Reactions
- Static and Kinetic Friction