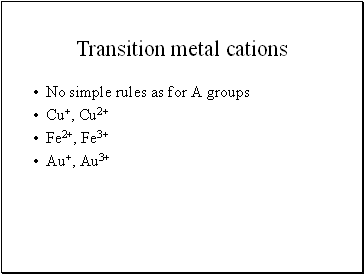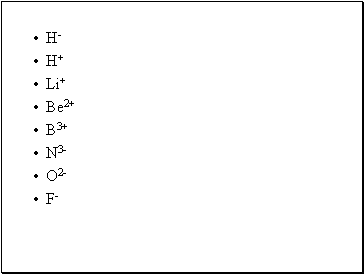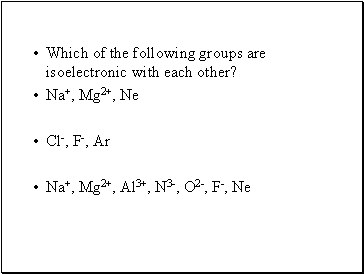Periodictable - QuestionsPage
5
5
Slide 41
Where do you get the numerical value for the n for the valence electrons?
You find the _ number!!!
Can you use this information to make electron configuration easier?
Slide 42
Valence electron configuration for:
P
Bi
Sr
Te
I
Cs
Slide 43
The octet rule
It has been noted that extra stability occurs when an atom or ion has 8 electrons in the outermost energy level (2 or 0 for the first period).
Slide 44
Group IA ns1
Lose
Group IIA ns2
Loses
Group IIIA ns2np1
Loses
Group IVA ns2np2
Group VA ns2np3
Gains
Group VIA ns2np4
Gains
Group VIIA ns2np5
Gains
Group VIIIA ns2np6
Slide 45
Group IA
Group IIA
Group IIIA
Group VA
Group VIA
Groupr VIIA
Names of ions: for cations--name of element plus ion
For anions: replace the last syllables of the element name by --ide + ion.
Slide 46
Transition metal cations
No simple rules as for A groups
Cu+, Cu2+
Fe2+, Fe3+
Au+, Au3+
Slide 47
H-
H+
Li+
Be2+
B3+
N3-
O2-
F-
Slide 48
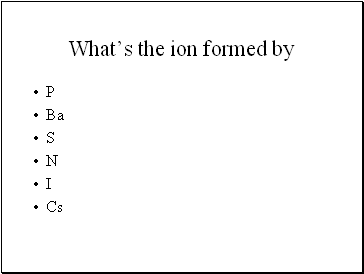
What’s the ion formed by
P
Ba
S
N
I
Cs
Slide 49
Slide 50
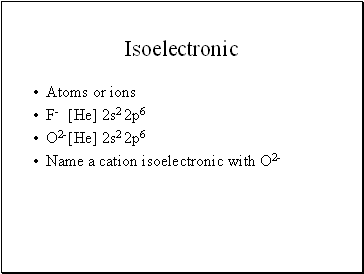
Isoelectronic
Atoms or ions
F- [He] 2s2 2p6
O2- [He] 2s2 2p6
Name a cation isoelectronic with O2-
Slide 51
Question 3.12
Which of the following pairs of atoms and ions are isoelectronic?
Cl-, Ar
Na+, Ne
Mg2+, Na+
Li+, Ne
O2-, F-
Slide 52
Which of the following groups are isoelectronic with each other?
Na+, Mg2+, Ne
Cl-, F-, Ar
Na+, Mg2+, Al3+, N3-, O2-, F-, Ne
Slide 53
Trends in the periodic table
Think of atom as sphere whose radius is determined by the location of the e’s furthest from the nucleus.
Contents
- Elements, atoms, ions, and the periodic table
- The periodic law and the periodic table
- Early periodic tables
- Modern periodic table
- Metals and nonmetals
- More info from periodic table
- Electron arrangement and the periodic table
- Principal energy levels (shells)
- Sublevels
- Orbitals
- Electron spin
- What to do with all this info?
- Abbreviated electron configuration
- Valence electrons
- Valence electron configuration for A groups
- The octet rule
- Transition metal cations
- What’s the ion formed by
- Isoelectronic
- Trends in the periodic table
- Size across a period
- Ion size
- Ionization energy
- Trends in ionization energy
- Electron affinity
- Trends in electron affinities
Last added presentations
- Static and Kinetic Friction
- Newton’s Law of Gravity
- Sound
- Geophysical Concepts, Applications and Limitations
- Resource Acquisition and Transport in Vascular Plants
- The Effects of Radiation on Living Things
- History of Modern Astronomy