Atomic TheoryPage
2
2
Slicing pepperoni for the topping
Spreading cheese on the pizza
Baking the dough to form the crust
Slide 11
What is the smallest particle of the element gold (Au) that can still be classified as gold?
atom
molecule
neutron
proton
Slide 12
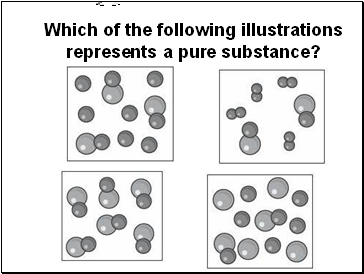
Which of the following illustrations represents a pure substance?
Slide 13
If 1 kg of the compound toluene melts at –95°C, then 500 g of toluene will
melt at –47.5°C.
melt at –95°C.
boil at 95°C.
boil at 47.5°C.
Slide 14
Slide 15
Which statement about the molecules in ice and the molecules in liquid water is correct?
The molecules in ice have more energy than the molecules in liquid water.
The molecules in ice contain different atoms than the molecules in liquid water.
The molecules in ice have more electric charge than the molecules in liquid water.
The molecules in ice are less free to move than the molecules in liquid water.
Slide 16
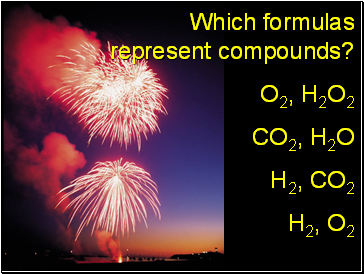
Which formulas represent compounds?
O2, H2O2
CO2, H2O
H2, CO2
H2, O2
Slide 17
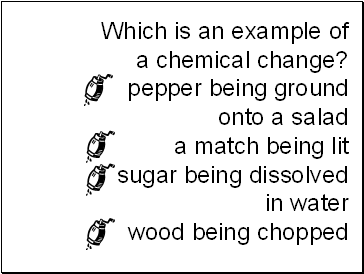
Which is an example of a chemical change?
pepper being ground onto a salad a match being lit sugar being dissolved in water wood being chopped
Slide 18
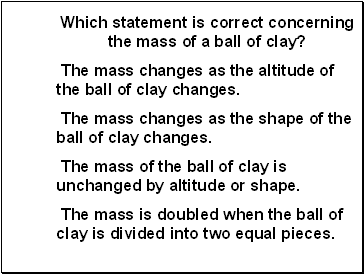
Which statement is correct concerning the mass of a ball of clay?
The mass changes as the altitude of the ball of clay changes.
The mass changes as the shape of the ball of clay changes.
The mass of the ball of clay is unchanged by altitude or shape.
The mass is doubled when the ball of clay is divided into two equal pieces.
Slide 19
Mary wants to find the density of a small stone. Which tools will she need?
a meterstick and a thermometer a thermometer and a balance a balance and a graduated cylinder a graduated cylinder and a meterstick
Slide 20
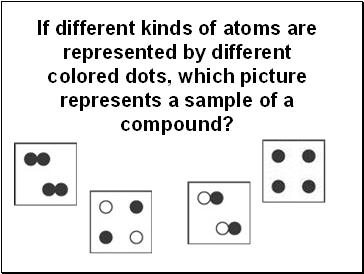
If different kinds of atoms are represented by different colored dots, which picture represents a sample of a compound?
Slide 21
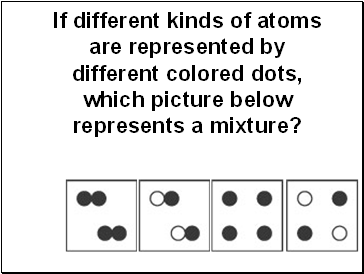
If different kinds of atoms are represented by different colored dots, which picture below represents a mixture?
Contents
- Ionic bond
- Ion
- Cations
- Non-Metals
- Metalloids
- States of Matter
- Neon
- Valence Electrons
- Isotopes
- Electron Shells
- Atomic Number
- Hydrogen
- Helium
- Lithium
- Beryllium
- Boron
- Carbon
- Nitrogen
- Oxygen
- Fluorine
- Element
- Which of the following is a compound?
- Which symbol represents carbon?
- Particle accelerator
- Proton
- Neutron
- Electromagnetic radiation
- Which formulas represent compounds?
- Which is an example of a chemical change?
- Which statement is correct concerning the mass of a ball of clay?
- Mary wants to find the density of a small stone. Which tools will she need?
- Forces
- Particles
- Which pair of elements is MOST similar?
- Anions
- Solution
- Covalent bond
- Common chemicals
- Combustibility
- Reaction Types
- Precipitate
- Balancing equations
- Distilled water
Last added presentations
- Upcoming Classes
- Health Physics
- The Effects of Radiation on Living Things
- Newton’s third law of motion
- Newton's Laws
- Newton’s law of universal gravitation
- Solar Energy