Atomic TheoryPage
9
9
H+ Hydrogen
Na+ Sodium
Mg+2 Magnesium
Ca+2 Calcium
Ag+2 Silver
Slide 124
Fe+2 Iron (II) Ferrous
Fe+3 Iron (III) Ferric
Cu+1 Copper (I) Cuprous
Cu+2 Copper (II) Cupric
NH4+ Ammonium
mo’ Cations
Slide 125
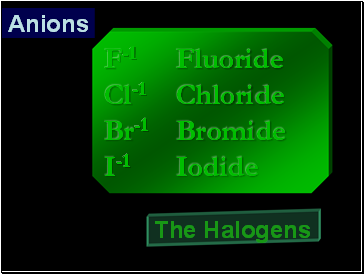
Anions
F-1 Fluoride
Cl-1 Chloride
Br-1 Bromide
I-1 Iodide
The Halogens
Slide 126
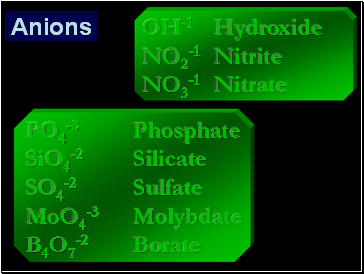
PO4-3 Phosphate
SiO4-2 Silicate
SO4-2 Sulfate
MoO4-3 Molybdate
B4O7-2 Borate
Anions
OH-1 Hydroxide
NO2-1 Nitrite
NO3-1 Nitrate
Slide 127
Cathode Anode
NeverReady
+
-
Slide 128
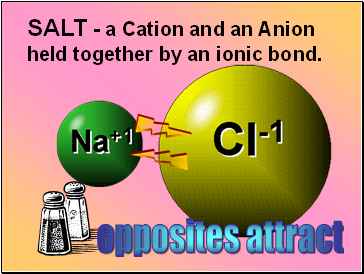
SALT - a Cation and an Anion held together by an ionic bond.
opposites attract
Slide 129
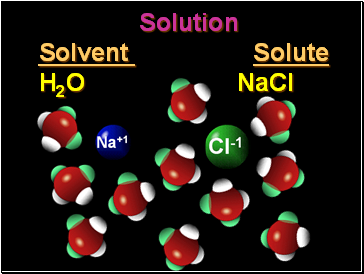
Solution
Solvent Solute
H2O NaCl
Slide 130
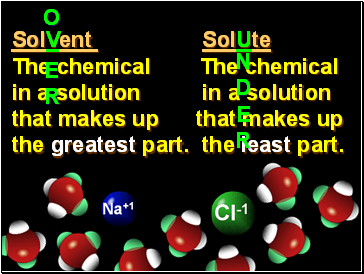
SolVent SolUte
The chemical The chemical
in a solution in a solution
that makes up that makes up
the greatest part. the least part.
O
E
R
N
DE
R
Slide 131
Sol ent
The chemical
in a solution
that makes up
the greatest part.
V
Slide 132
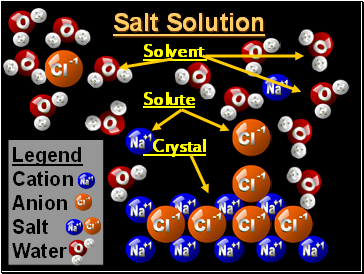
Salt Solution
Legend
Cation
Anion
Salt
Water
Solvent
Solute
Crystal
Slide 133
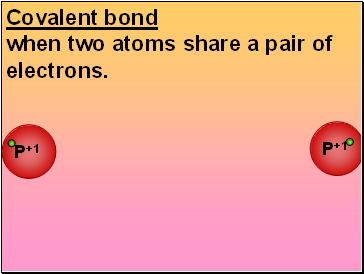
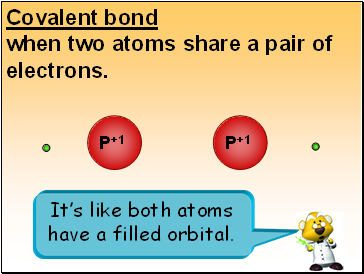
Covalent bond
P+1
when two atoms share a pair of electrons.
P+1
Slide 134
Covalent bond
when two atoms share a pair of electrons.
P+1
P+1
It’s like both atoms have a filled orbital.
Slide 135
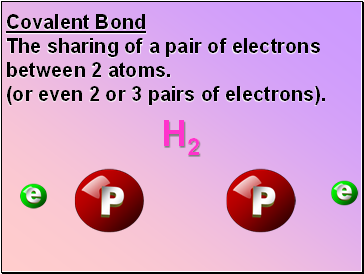
Covalent Bond
The sharing of a pair of electrons between 2 atoms.
(or even 2 or 3 pairs of electrons).
H2
Slide 136
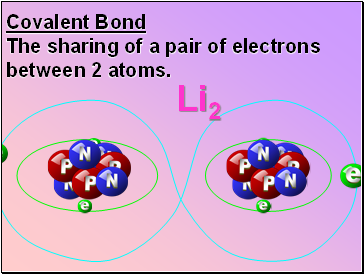
Covalent Bond
The sharing of a pair of electrons between 2 atoms.
Li2
Slide 137
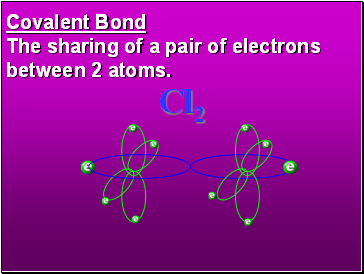
Covalent Bond
The sharing of a pair of electrons between 2 atoms.
Cl2
Slide 138
Contents
- Ionic bond
- Ion
- Cations
- Non-Metals
- Metalloids
- States of Matter
- Neon
- Valence Electrons
- Isotopes
- Electron Shells
- Atomic Number
- Hydrogen
- Helium
- Lithium
- Beryllium
- Boron
- Carbon
- Nitrogen
- Oxygen
- Fluorine
- Element
- Which of the following is a compound?
- Which symbol represents carbon?
- Particle accelerator
- Proton
- Neutron
- Electromagnetic radiation
- Which formulas represent compounds?
- Which is an example of a chemical change?
- Which statement is correct concerning the mass of a ball of clay?
- Mary wants to find the density of a small stone. Which tools will she need?
- Forces
- Particles
- Which pair of elements is MOST similar?
- Anions
- Solution
- Covalent bond
- Common chemicals
- Combustibility
- Reaction Types
- Precipitate
- Balancing equations
- Distilled water
Last added presentations
- Sound
- Static and Kinetic Friction
- Waves & Sound
- Newton's laws of motion
- Resource Acquisition and Transport in Vascular Plants
- Ch 9 Nuclear Radiation
- Soil and Plant Nutrition