AtomsPage
4
4
Concerned about radioactivity in nature?
To keep things in perspective, consider that 0.01% of all potassium is radioactive K-40.
Potassium is an essential element in the human body.
If your body is about 1% K, this means a 70 kg
(150 pound) person contains around
1x1021 atoms (thatís one billion trillion atoms)
of radioactive K-40.
Slide 21
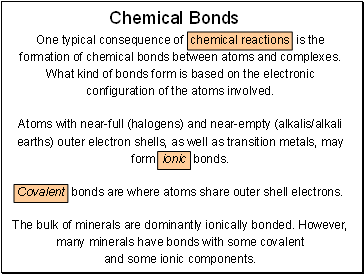
Chemical Bonds
One typical consequence of chemical reactions is the formation of chemical bonds between atoms and complexes.
What kind of bonds form is based on the electronic configuration of the atoms involved.
Atoms with near-full (halogens) and near-empty (alkalis/alkali earths) outer electron shells, as well as transition metals, may form ionic bonds.
Covalent bonds are where atoms share outer shell electrons.
The bulk of minerals are dominantly ionically bonded. However, many minerals have bonds with some covalent
and some ionic components.
Slide 22
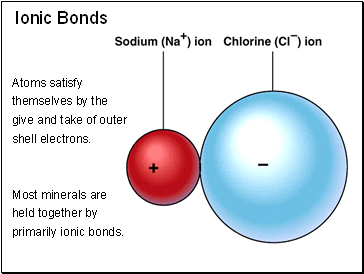
Ionic Bonds
Atoms satisfy themselves by the give and take of outer shell electrons.
Most minerals are held together by primarily ionic bonds.
Slide 23
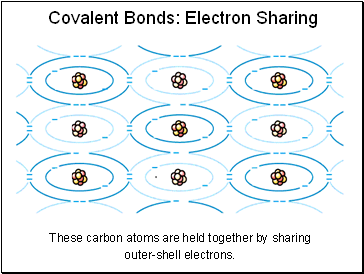
Covalent Bonds: Electron Sharing
These carbon atoms are held together by sharing outer-shell electrons.
Slide 24
Alternative Bonds
Some minerals have metallic bonds,
which are a form of covalent bonds.
One notable weak kind of bond is called the
Van der Waals force, essentially an ionic bond.
When we talk about minerals we will see that these bonds are responsible for the key
physical properties of some minerals.
Slide 25
Chemical Reactions: Achieving Stability
Chemical reactions take place in order to achieve a more stable state (lower total energy) under given conditions (pressure, temperature).
Unstable reactants react to form stable products.
[donít get confused: weíre not talking about nuclear instability here]
To complicate this, the transition from unstable mineral to stable mineral is not necessarily automatic.
Many chemical reactions require
a great deal of energy
to run to completion.
Slide 26
What is a Mineral?
naturally occurring
inorganic compound
specific chemical composition
defined crystal structure
consistent physical properties
Slide 27
Stability
With sophisticated furnaces and presses, we have investigated what chemical compounds (minerals) are stable at given pressures and temperatures
Contents
- The Building Blocks
- The Stuff That Makes Atoms
- Maintaining Neutrality
- Electronic and Nuclear Properties
- Size of Nuclei
- The Spacious Atom
- Electrons in Orbit
- Charged Atoms: Ions
- Atomic Number
- The Periodic Table
- Oxidation State
- Oxidized and Reduced States
- The Periodic Table: the Bulk Earth
- The Periodic Table: the Crust
- Atomic Weight: Itís all in the Nucleus
- Isotopes
- Radioactive or Stable?
- Stable and Radioactive Isotopes
- Radioactivity Inside You
- Chemical Bonds
- Ionic Bonds
- Covalent Bonds: Electron Sharing
- Alternative Bonds
- Chemical Reactions: Achieving Stability
- What is a Mineral?
- Stability
- Stability and Metastability
Last added presentations
- Mechanics Lecture
- The Effects of Radiation on Living Things
- Geophysical Concepts, Applications and Limitations
- Friction
- Sound
- Radiation
- Waves & Sound