Chemical Bonding revisedPage
2
2
So far we have seen molecules represented in 2-D
However, molecules are actually 3 dimensional
To predict 3 dimensional molecular shapes we use VSEPR theory (Valence-Shell Electron-Pair Repulsion)
Based on the electrostatic repulsion of electron pairs
Slide 11
Note that the repulsion force is strongest between two lone pairs and the weakest between two bonded pairs, and the repulsion between a lone pair and a bonded pair is intermediate
We apply the VSEPR theory to a central atom that has an octet of electrons in its valence shell, and there are three categories of shapes; linear, trigonal planar, and tetrahedral
In VSEPR, an electron group is a bond (single or multiple) or a lone pair
Slide 12
Linear
Forms when a central atom has two electron groups
The shape is linear because the electron groups try to arrange themselves as far apart as possible
The bond angle between the electron groups is 1800
The central atom is bonded to two other atoms by two double bonds or a combination of a single bond and a triple bond
Slide 13
Trigonal Planar
A central atom with three electron groups has a trigonal planar shape
The bonding angle between the electron groups is 120o
The central atom is either bonded to three atoms(trigonal planar), or two atoms and a lone pair(bent or V-shaped)
Slide 14
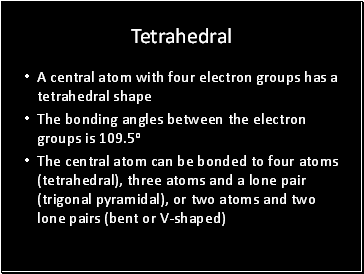
Tetrahedral
A central atom with four electron groups has a tetrahedral shape
The bonding angles between the electron groups is 109.5o
The central atom can be bonded to four atoms (tetrahedral), three atoms and a lone pair (trigonal pyramidal), or two atoms and two lone pairs (bent or V-shaped)
Slide 15
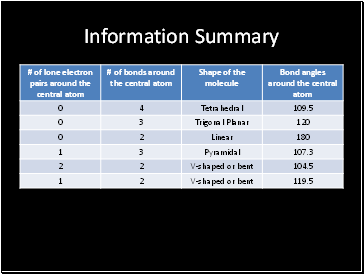
Information Summary
Slide 16
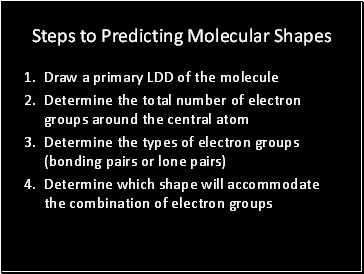
Steps to Predicting Molecular Shapes
Draw a primary LDD of the molecule
Determine the total number of electron groups around the central atom
Determine the types of electron groups (bonding pairs or lone pairs)
Determine which shape will accommodate the combination of electron groups
Slide 17
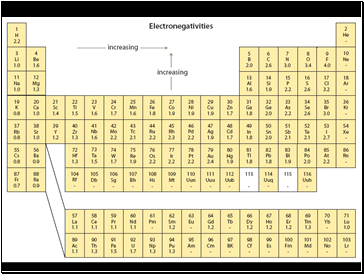
Electronegativity
Electronegativity is a measure of the relative ability of an element’s atoms to attract the shared electrons in a chemical bond.
Higher electronegativities mean a greater attraction for the electrons.
Fluorine is the highest with a value of 4.0
Slide 18
Contents
- Covalent Bonding
- Lewis Dot Diagrams
- Multiple Bonds
- Multiple Lewis Structures
- Structural Diagrams
- Lewis Dot Diagram Worksheet
- Stereochemistry – The Structures of Molecular Compounds
- Linear
- Trigonal Planar
- Tetrahedral
- Steps to Predicting Molecular Shapes
- Electronegativity
- Atom Size
- Polarity
- Polar Molecules
- Ionic Bonds
- Metallic Bonding
- Ionic Crystals
- Crystal formation
- Network Solids
- Intermolecular Forces
- Dipole-Dipole Forces
- Hydrogen Bonding
- Hydrogen Bonding in Water
- Hydrogen Bonds in Ice
- Unique Properties Reading
- London Dispersion Forces
- Factors Affecting Magnitude
- Structures and Properties of Compounds
- Time of Hydrogen Bonding
- Melting and Boiling Points
- Molecular Forces
- Mechanical Properties of Solids
- Conductivity
Last added presentations
- Solar Thermal Energy
- Gravitation
- Radiation Safety and Operations
- Practical Applications of Solar Energy
- Thermal Energy
- History of Modern Astronomy
- Static and Kinetic Friction