Chemical Bonding revisedPage
4
4
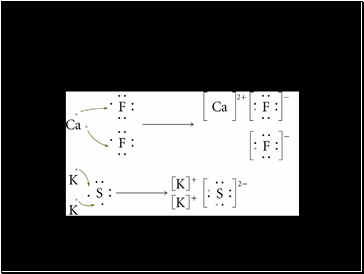
Electron Exchange and Ionic Bond Formation
Slide 30
Metallic Bonding
Metals can form bonds with other metals, but it is neither covalent or ionic
Metals cannot share electrons to form an octet of electrons around each atom
Imagine 8 sodium atoms all trying to share the same 8 electrons
Slide 31
Although metal atoms do not form covalent or ionic bonds with each other, there must be relatively strong attractive forces holding the atoms together or else the metals would be in a gaseous state
In metallic bonding, the valence electrons are delocalized, which means they are free to move from one atom to the next
Slide 32
Because the electrons are free to move, all of the atoms share all of the valence electrons
It is the electrostatic force between the positively charged metal ions and the negative electrons that make the metallic bond
Slide 33
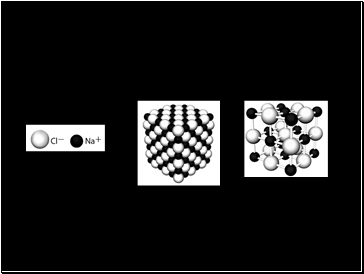
Ionic Crystals
Rather than one metal bonding to one non-metal, ionic substances have their ions packed together in a crystal lattice
The crystals can also be represented in a ball and stick model
Slide 34
Slide 35
The sticks represent the attractive forces between the ions
Since all the attractions are equal, there are no pairs of ions to be identified as molecules
Therefore the formula only represents the ratio of the ions in the crystal
Slide 36
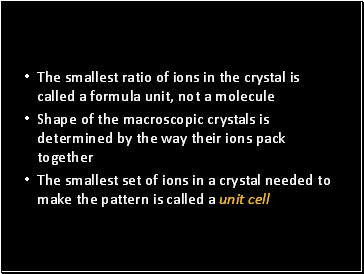
The smallest ratio of ions in the crystal is called a formula unit, not a molecule
Shape of the macroscopic crystals is determined by the way their ions pack together
The smallest set of ions in a crystal needed to make the pattern is called a unit cell
Slide 37
Table Salt Sucrose Uncut Diamond
Slide 38
The size of each ion influences the pattern of ions
Another influence is the relative charge of the ions, and therefore the ratio of ions in the crystal
Slide 39
Crystal formation
Many beautiful crystal formations can be found in nature as well as in the laboratory
Slide 40
Contents
- Covalent Bonding
- Lewis Dot Diagrams
- Multiple Bonds
- Multiple Lewis Structures
- Structural Diagrams
- Lewis Dot Diagram Worksheet
- Stereochemistry – The Structures of Molecular Compounds
- Linear
- Trigonal Planar
- Tetrahedral
- Steps to Predicting Molecular Shapes
- Electronegativity
- Atom Size
- Polarity
- Polar Molecules
- Ionic Bonds
- Metallic Bonding
- Ionic Crystals
- Crystal formation
- Network Solids
- Intermolecular Forces
- Dipole-Dipole Forces
- Hydrogen Bonding
- Hydrogen Bonding in Water
- Hydrogen Bonds in Ice
- Unique Properties Reading
- London Dispersion Forces
- Factors Affecting Magnitude
- Structures and Properties of Compounds
- Time of Hydrogen Bonding
- Melting and Boiling Points
- Molecular Forces
- Mechanical Properties of Solids
- Conductivity
Last added presentations
- Practical Applications of Solar Energy
- Radiation Safety and Operations
- Space Radiation
- Upcoming Classes
- Heat-Energy on the Move
- Gravitation
- Solar Energy