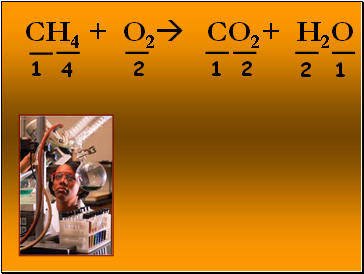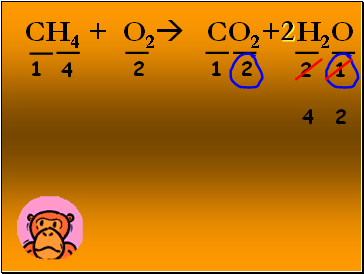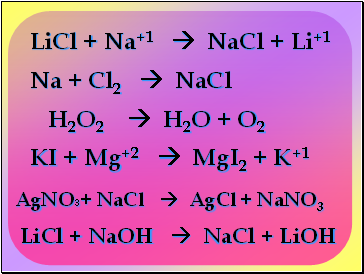ChemistryPage
9
9
pH paper
Blue Litmus paper
Red Litmus paper
Slide 100
Balancing equations
Slide 101
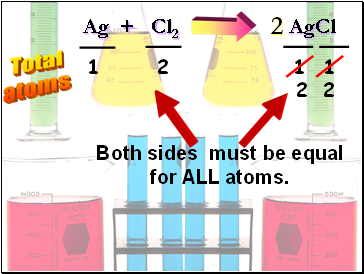
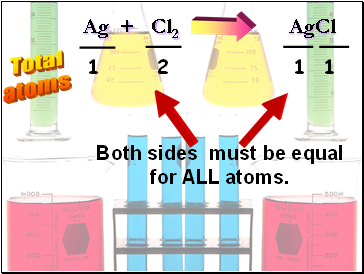
Ag + Cl2 AgCl
Total
atoms
1
2
1
1
Both sides must be equal
for ALL atoms.
2
2
2
Slide 102
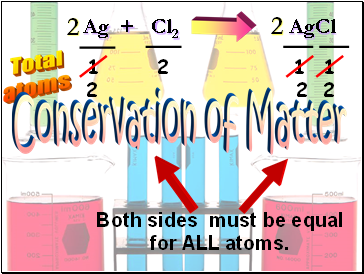
Ag + Cl2 AgCl
Total
atoms
1
2
1
1
Both sides must be equal
for ALL atoms.
2
2
2
2
2
Conservation of Matter
Slide 103
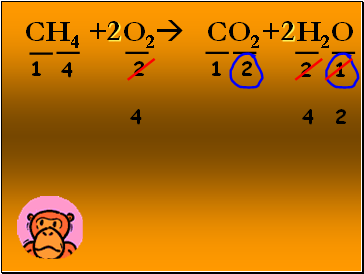
CH4 + O2 CO2+ H2O
1
2
1
2
4
2
1
Slide 104
CH4 + O2 CO2+ H2O
1
2
1
2
4
2
1
2
2
4
Slide 105
CH4 + O2 CO2+ H2O
1
2
1
2
4
2
1
2
2
4
2
4
Slide 106
Ag + Cl2 AgCl
Total
atoms
1
2
1
1
Both sides must be equal
for ALL atoms.
Slide 107
LiCl + Na+1 NaCl + Li+1
Na + Cl2 NaCl
H2O2 H2O + O2
KI + Mg+2 MgI2 + K+1
AgNO3+ NaCl AgCl + NaNO3
LiCl + NaOH NaCl + LiOH
Slide 108
Catalyst cat a list
a substance which alters the rate of a chemical reaction.
It is unchanged at the end of the reaction.
Slide 109
Experiment with the changes of states of a substance (i.e. water, dry ice).
Chart- state changes: melting, boiling, freezing, evaporation, condensation, and sublimation.
Slide 110
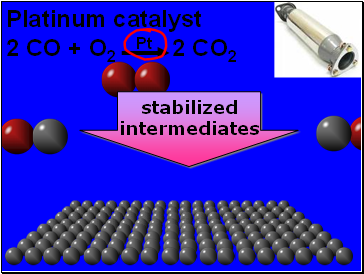
Platinum catalyst
2 CO + O2 Pt 2 CO2
stabilized
intermediates
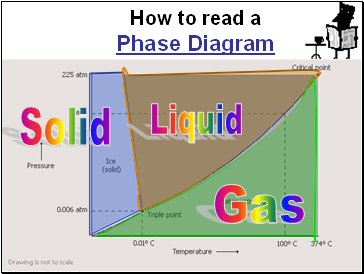
Slide 111
How to read a
Phase Diagram
Solid
Gas
Liquid
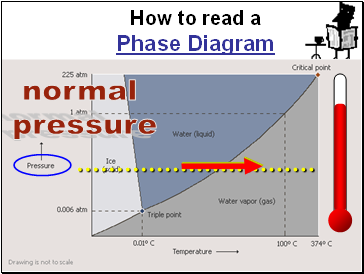
Slide 112
How to read a
Phase Diagram
normal
pressure
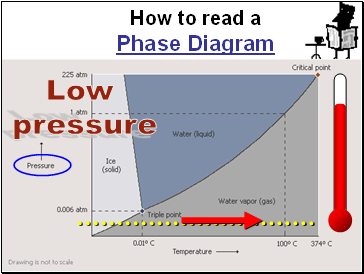
Slide 113
How to read a
Phase Diagram
Low
pressure
Slide 114
Contents
- Reaction Types
- Chemical Bonds
- Ionic bond
- Cations
- Anions
- Which is a metric unit for density?
- When a gas forms a liquid, which process is taking place?
- Which unit correctly describes density?
- Based on the melting points shown in the table, which material would still be a solid at 400°C?
- A chemical change for a piece of metal would be
- Which symbolizes a molecule of a compound?
- Putting sand and salt together makes
- Plastic, wood, and iron are all made up of
- An atom is to an element, as a molecule is to a
- Which is the correct symbol for the element sodium?
- Covalent bond
- Reactivity
- Calorimeter
- Combustibility
- Biochemicals
- Sugars
- Wawa
Last added presentations
- Mechanics Lecture
- Radioactivity and Nuclear Reactions
- Space Radiation
- Simulation at NASA for the Space Radiation Effort
- Ch 9 Nuclear Radiation
- Thermal Energy
- Geophysical Concepts, Applications and Limitations