Rutherford ScatteringPage
2
2
a
a
a
a
a
a
a
Slide 20
a
a
a
a
a
a
a
Slide 21
a
a
a
a
a
a
a
Slide 22
a
a
a
a
a
a
a
Slide 23
a
a
a
a
a
a
a
Slide 24
a
a
a
a
a
a
a
Slide 25
a
a
a
a
a
a
a
Slide 26
a
a
a
a
a
a
a
Slide 27
a
a
a
a
a
a
Slide 28
a
a
a
a
a
a
Slide 29
a
a
a
a
a
a
Slide 30
Results
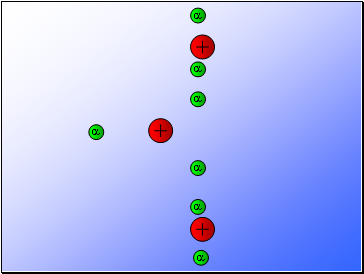
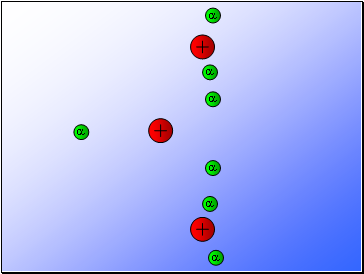
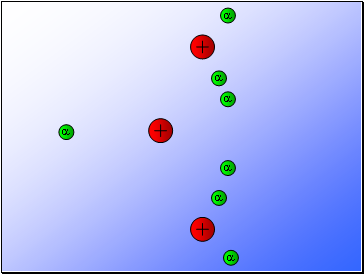
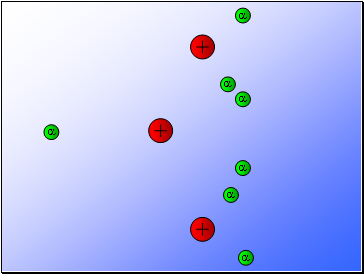
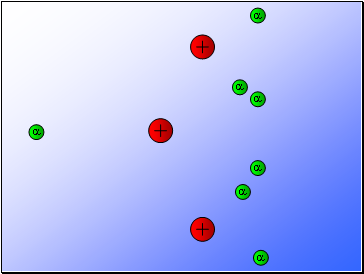
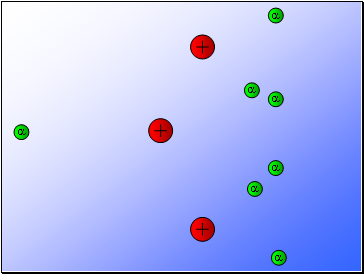
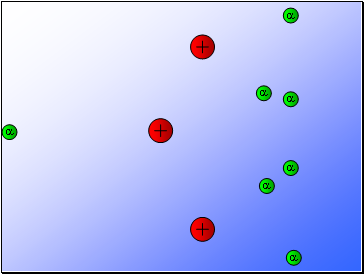
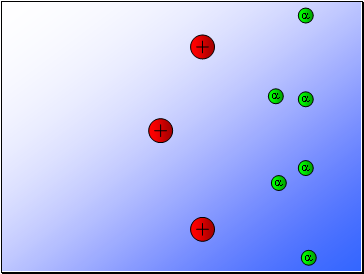
Rutherford’s experiment found that:
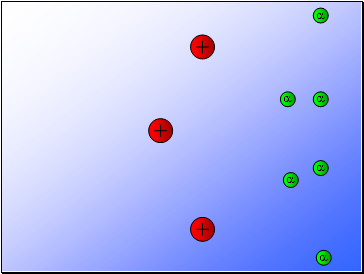
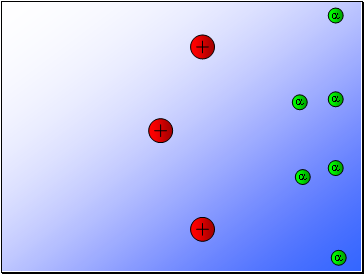
Most of the alpha particles passed through the gold foil
undeviated.
A few alpha particles were deflected from their path
but continued through the gold foil.
A small number of alpha particles rebounded.
Slide 31
Conclusion
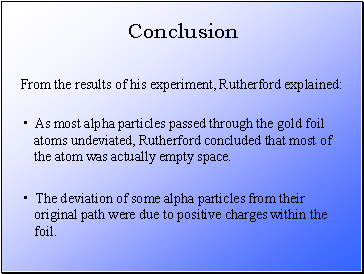
As most alpha particles passed through the gold foil
atoms undeviated, Rutherford concluded that most of
the atom was actually empty space.
From the results of his experiment, Rutherford explained:
The deviation of some alpha particles from their
original path were due to positive charges within the
foil.
Slide 32
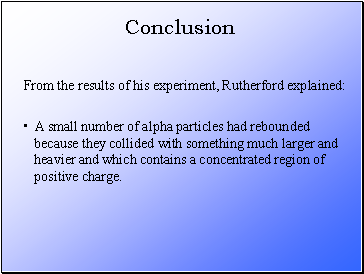
From the results of his experiment, Rutherford explained:
Conclusion
A small number of alpha particles had rebounded
because they collided with something much larger and
heavier and which contains a concentrated region of
positive charge.
Slide 33
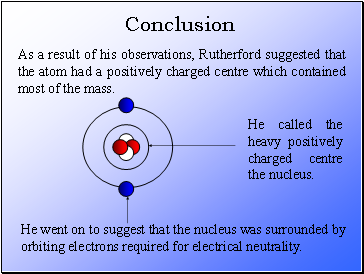
As a result of his observations, Rutherford suggested that the atom had a positively charged centre which contained most of the mass.
He called the heavy positively charged centre the nucleus.
He went on to suggest that the nucleus was surrounded by orbiting electrons required for electrical neutrality.
Contents
Last added presentations
- Radiation
- Direct heat utilization of geothermal energy
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Newton’s law of universal gravitation
- Heat-Energy on the Move
- History of Modern Astronomy
- Friction