Rutherford ScatteringPage
3
3
Conclusion
Slide 34
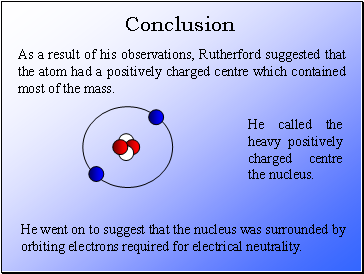
As a result of his observations, Rutherford suggested that the atom had a positively charged centre which contained most of the mass.
He called the heavy positively charged centre the nucleus.
He went on to suggest that the nucleus was surrounded by orbiting electrons required for electrical neutrality.
Conclusion
Slide 35
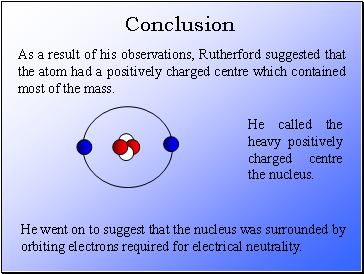
As a result of his observations, Rutherford suggested that the atom had a positively charged centre which contained most of the mass.
He called the heavy positively charged centre the nucleus.
He went on to suggest that the nucleus was surrounded by orbiting electrons required for electrical neutrality.
Conclusion
Slide 36
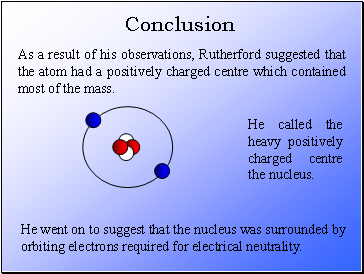
As a result of his observations, Rutherford suggested that the atom had a positively charged centre which contained most of the mass.
He called the heavy positively charged centre the nucleus.
He went on to suggest that the nucleus was surrounded by orbiting electrons required for electrical neutrality.
Conclusion
Slide 37
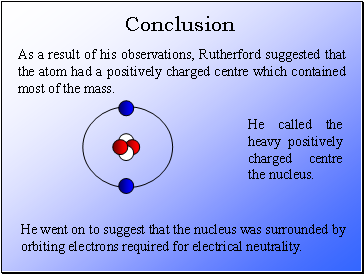
As a result of his observations, Rutherford suggested that the atom had a positively charged centre which contained most of the mass.
He called the heavy positively charged centre the nucleus.
He went on to suggest that the nucleus was surrounded by orbiting electrons required for electrical neutrality.
Conclusion
Slide 38
Modern measurements show that the average nucleus has a radius in the order of 10-15 m. This is 100, 000 times smaller than the radius of a typical atom.
Contents
Last added presentations
- Waves & Sound
- Thermal Energy
- Newton’s law of universal gravitation
- Soil and Plant Nutrition
- Sensory and Motor Mechanisms
- Newton's Laws
- Health Physics