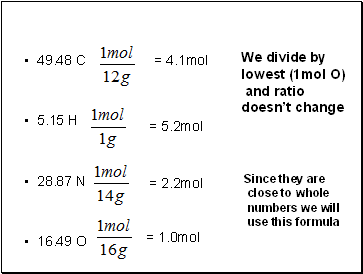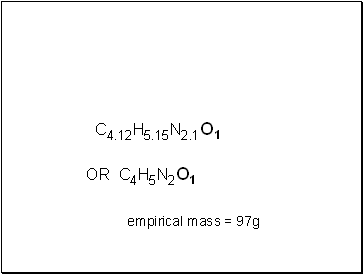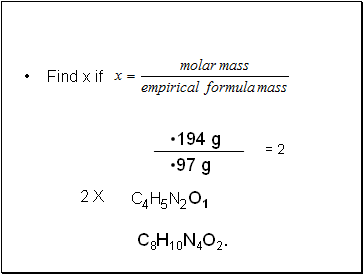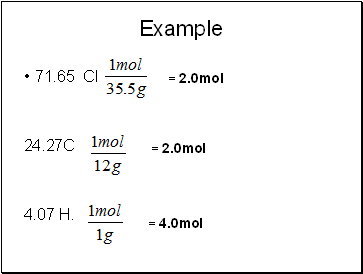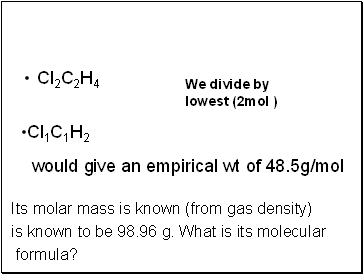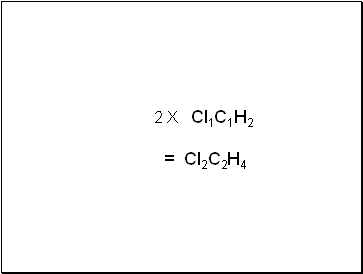Empirical FormulaPage
2
2
Caffeine is 49.48% C, 5.15% H, 28.87% N and 16.49% O. What is its empirical formula?
Slide 12
49.48 C
5.15 H
28.87 N
16.49 O
= 4.1mol
= 5.2mol
= 2.2mol
= 1.0mol
Since they are
close to whole
numbers we will
use this formula
We divide by
lowest (1mol O)
and ratio
doesn’t change
Slide 13
C4.12H5.15N2.1O1
empirical mass = 97g
OR C4H5N2O1
Slide 14
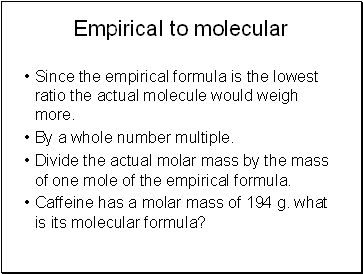
Empirical to molecular
Since the empirical formula is the lowest ratio the actual molecule would weigh more.
By a whole number multiple.
Divide the actual molar mass by the mass of one mole of the empirical formula.
Caffeine has a molar mass of 194 g. what is its molecular formula?
Slide 15
Find x if
194 g
97 g
= 2
C4H5N2O1
C8H10N4O2.
2 X
Slide 16
Example
A compound is known to be composed of 71.65 % Cl, 24.27% C and 4.07% H. Its molar mass is known (from gas density) is known to be 98.96 g. What is its molecular formula?
Slide 17
Example
71.65 Cl
24.27C
4.07 H.
= 2.0mol
= 2.0mol
= 4.0mol
Slide 18
Cl2C2H4
Its molar mass is known (from gas density)
is known to be 98.96 g. What is its molecular
formula?
We divide by
lowest (2mol )
Cl1C1H2
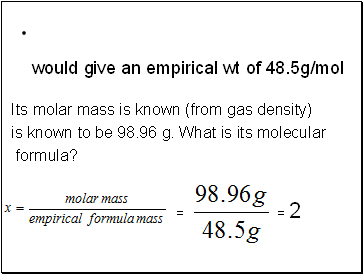
would give an empirical wt of 48.5g/mol
Slide 19
Its molar mass is known (from gas density)
is known to be 98.96 g. What is its molecular
formula?
would give an empirical wt of 48.5g/mol
= 2
=
Slide 20
2 X Cl1C1H2
= Cl2C2H4
1 2
Contents
Last added presentations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Newton’s laws of motion
- Simulation at NASA for the Space Radiation Effort
- Newton's Laws
- Thermal Energy
- Health Physics
- Resource Acquisition and Transport in Vascular Plants