Strengths of Acids and Bases Making DilutionsPage
2
2
New molarity = 0.15 moles = 1.5 M
0.100 L
Slide 11
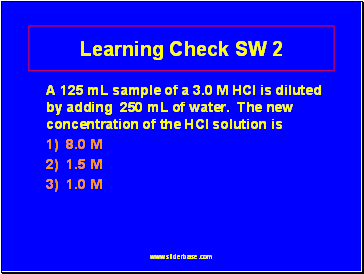
Learning Check SW 2
A 125 mL sample of a 3.0 M HCl is diluted by adding 250 mL of water. The new concentration of the HCl solution is
1) 8.0 M
2) 1.5 M
3) 1.0 M
Slide 12
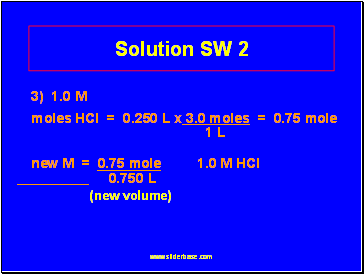
Solution SW 2
3) 1.0 M
moles HCl = 0.250 L x 3.0 moles = 0.75 mole
1 L
new M = 0.75 mole 1.0 M HCl
0.750 L
(new volume)
Slide 13
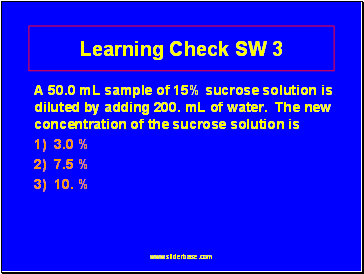
Learning Check SW 3
A 50.0 mL sample of 15% sucrose solution is diluted by adding 200. mL of water. The new concentration of the sucrose solution is
1) 3.0 %
2) 7.5 %
3) 10. %
Slide 14
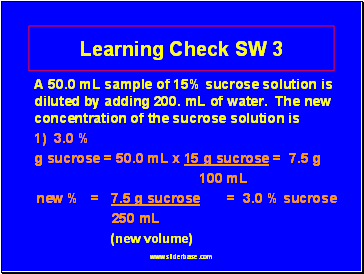
Learning Check SW 3
A 50.0 mL sample of 15% sucrose solution is diluted by adding 200. mL of water. The new concentration of the sucrose solution is
1) 3.0 %
g sucrose = 50.0 mL x 15 g sucrose = 7.5 g
100 mL
new % = 7.5 g sucrose = 3.0 % sucrose
250 mL
(new volume)
1 2
Contents
Last added presentations
- Soil and Plant Nutrition
- Solar Thermal Energy
- Radiation Safety and Operations
- Thermal Energy
- Sensory and Motor Mechanisms
- Newton’s laws of motion
- Radioactivity and Nuclear Reactions