Atoms and the Periodic tablePage
3
3
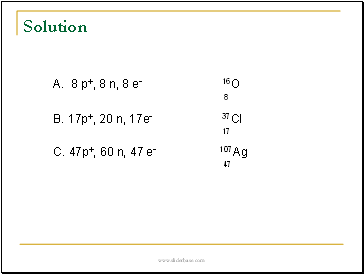
A. 8 p+, 8 n, 8 e- _
B. 17p+, 20n, 17e- _
C. 47p+, 60 n, 47 e- _
Slide 18
Slide 19
Learning Check
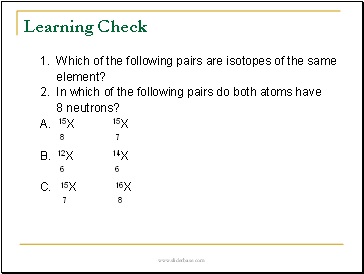
1. Which of the following pairs are isotopes of the same
element?
2. In which of the following pairs do both atoms have
8 neutrons?
A. 15X 15X
8 7
B. 12X 14X
6 6
C. 15X 16X
7 8
Slide 20
Solution
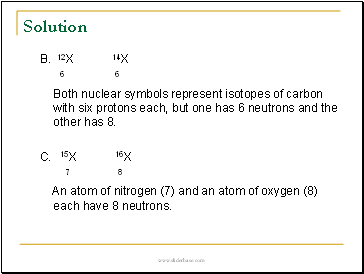
B. 12X 14X
6 6
Both nuclear symbols represent isotopes of carbon with six protons each, but one has 6 neutrons and the other has 8.
C. 15X 16X
7 8
An atom of nitrogen (7) and an atom of oxygen (8) each have 8 neutrons.
Slide 21
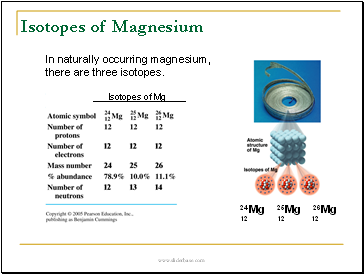
Isotopes of Magnesium
In naturally occurring magnesium,
there are three isotopes.
Slide 22
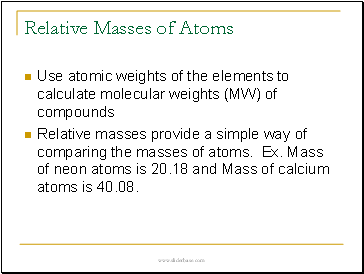
Relative Masses of Atoms
Use atomic weights of the elements to calculate molecular weights (MW) of compounds
Relative masses provide a simple way of comparing the masses of atoms. Ex. Mass of neon atoms is 20.18 and Mass of calcium atoms is 40.08.
Slide 23
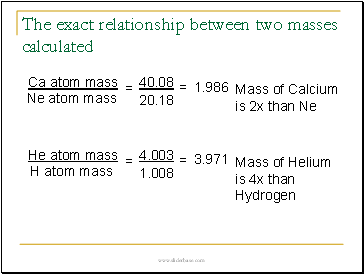
The exact relationship between two masses calculated
Slide 24
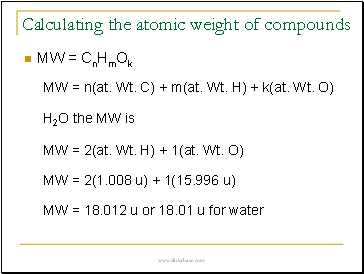
Calculating the atomic weight of compounds
Slide 25
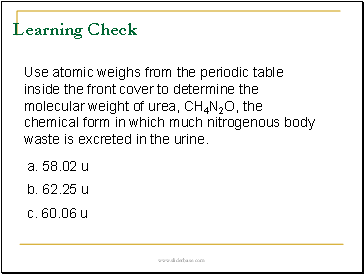
Learning Check
Use atomic weighs from the periodic table inside the front cover to determine the molecular weight of urea, CH4N2O, the chemical form in which much nitrogenous body waste is excreted in the urine.
a. 58.02 u
b. 62.25 u
c. 60.06 u
Slide 26
Solution
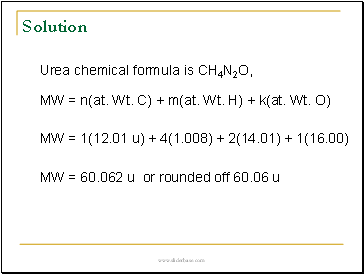
Urea chemical formula is CH4N2O,
MW = n(at. Wt. C) + m(at. Wt. H) + k(at. Wt. O)
MW = 1(12.01 u) + 4(1.008) + 2(14.01) + 1(16.00)
MW = 60.062 u or rounded off 60.06 u
Slide 27
Chapter Outline
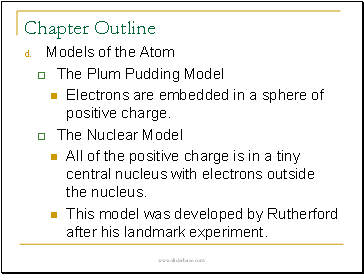
• Models of the Atom
The Plum Pudding Model
Electrons are embedded in a sphere of positive charge.
The Nuclear Model
All of the positive charge is in a tiny central nucleus with electrons outside the nucleus.
This model was developed by Rutherford after his landmark experiment.
Slide 28
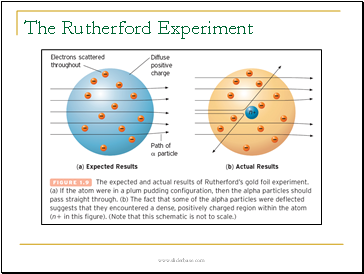
The Rutherford Experiment
Contents
- Atoms and the periodic table
- Conservation of Matter
- Atomic Line Spectra
- Atomic Subshell Energies
- Exploration of the Periodic Table/ Periodic Reactivity Trends
Last added presentations
- Newton’s Laws of Motion
- Magnetic field uses sound waves to ignite sun's ring of fire
- Newton’s third law of motion
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Newton’s laws of motion
- Thermal Energy
- Sound