Atoms and the Periodic tablePage
5
5
Slide 38
Chapter Outline
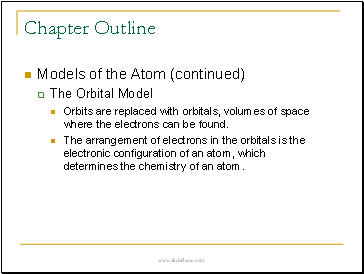
Models of the Atom (continued)
The Orbital Model
Orbits are replaced with orbitals, volumes of space where the electrons can be found.
The arrangement of electrons in the orbitals is the electronic configuration of an atom, which determines the chemistry of an atom.
Slide 39
Definitions
Electrons in the highest occupied energy level are the greatest stable distance from the nucleus. These outermost electrons are known as valence electrons.
Shell is a principal energy level defined by a given value of n, where n can be 1,2,3,4 etc… and is capable of holding 2n2 electrons.
An orbital is a region of three-dimensional space around an atom within which there is a significant probability (usually shown as 90%) that a given electron will be found.
Subshells have different energy levels (orbitals) within a given shell
Slide 40
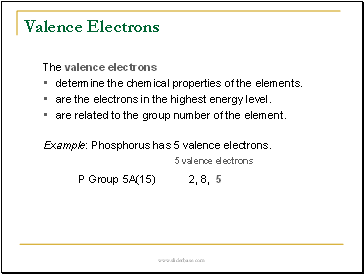
Valence Electrons
The valence electrons
•determine the chemical properties of the elements.
•are the electrons in the highest energy level.
•are related to the group number of the element.
Example: Phosphorus has 5 valence electrons.
5 valence electrons
P Group 5A(15) 2, 8, 5
Slide 41
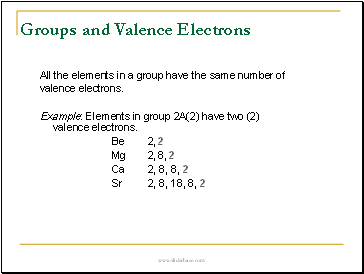
Groups and Valence Electrons
All the elements in a group have the same number of
valence electrons.
Example: Elements in group 2A(2) have two (2) valence electrons.
Be 2, 2
Mg 2, 8, 2
Ca 2, 8, 8, 2
Sr 2, 8, 18, 8, 2
Slide 42
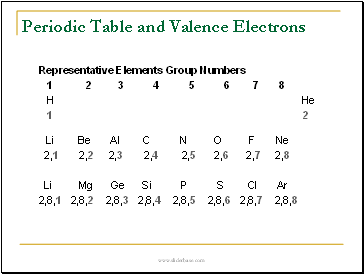
Periodic Table and Valence Electrons
Representative Elements Group Numbers
1 2 3 4 5 6 7 8
H He
1 2
Li Be Al C N O F Ne
2,1 2,2 2,3 2,4 2,5 2,6 2,7 2,8
Li Mg Ge Si P S Cl Ar
2,8,1 2,8,2 2,8,3 2,8,4 2,8,5 2,8,6 2,8,7 2,8,8
Slide 43
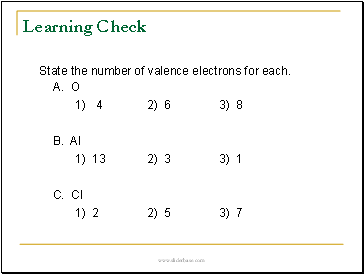
Learning Check
State the number of valence electrons for each.
A. O
1) 4 2) 6 3) 8
B. Al
1) 13 2) 3 3) 1
C. Cl
1) 2 2) 5 3) 7
Slide 44
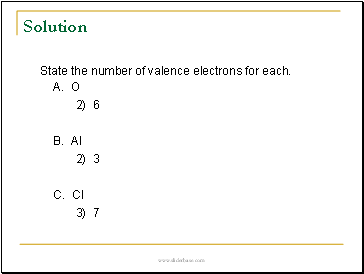
Solution
State the number of valence electrons for each.
A. O
2) 6
B. Al
2) 3
C. Cl
3) 7
Slide 45
Contents
- Atoms and the periodic table
- Conservation of Matter
- Atomic Line Spectra
- Atomic Subshell Energies
- Exploration of the Periodic Table/ Periodic Reactivity Trends
Last added presentations
- Solar Thermal Energy
- Radiation Safety and Operations
- Buoyancy
- Solar Energy
- Madame Marie Curie
- Radioactivity and Nuclear Reactions
- Magnetic field uses sound waves to ignite sun's ring of fire