Ch 9 Nuclear RadiationPage
1
1
Slide 1
Ch 9 - Nuclear Radiation
Nuclear Emissions
Nuclear Equations
Producing Radioactive Isotopes
Half-Life
Nuclear Fission and Fusion
Uses & Effects
Slide 2
Review
Remember
Protons: + charge
Neutrons: neutral
Electrons: - charge
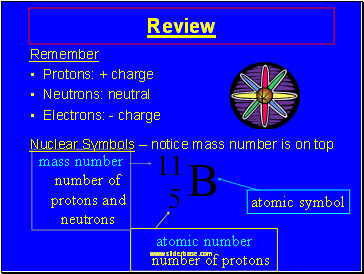
atomic symbol
atomic number number of protons
mass number number of protons and neutrons
Nuclear Symbols – notice mass number is on top
Slide 3
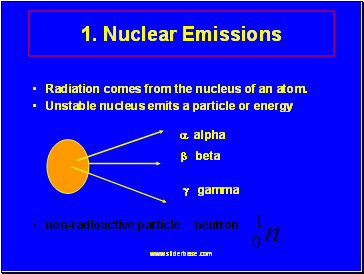
Nuclear Emissions
Radiation comes from the nucleus of an atom.
Unstable nucleus emits a particle or energy alpha
beta
gamma
non-radioactive particle = neutron
Slide 4
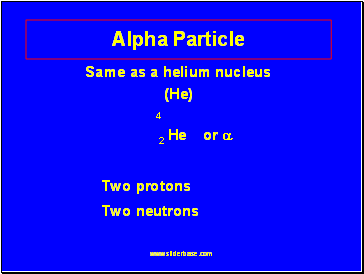
Alpha Particle
Same as a helium nucleus
(He)
4
2 He or
Two protons
Two neutrons
Slide 5
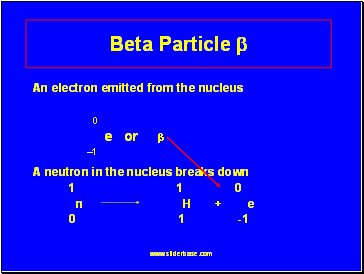
Beta Particle
An electron emitted from the nucleus
0
e or
1
A neutron in the nucleus breaks down
1 1 0
n H + e
0 1 -1
Slide 6
Gamma Radiation
Pure radiation
Like an X-ray but comes from the nucleus
Slide 7
Radiation Protection
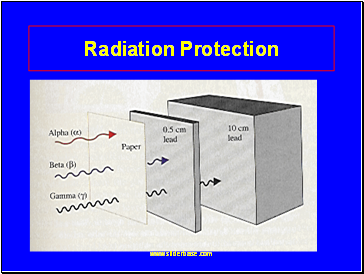
Shielding
alpha – paper, clothing
beta – lab coat, gloves
gamma- lead, thick concrete
Limit time exposed
Keep distance from source
Slide 8
Radiation Protection
Slide 9
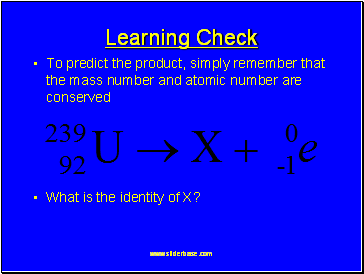
Nuclear Equations
In the reactants and products
Atomic numbers must balance
and
Mass numbers must balance
Slide 10
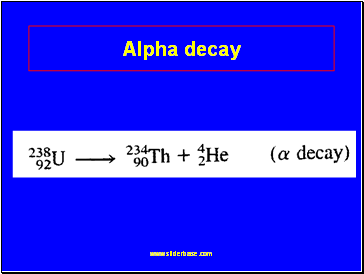
Alpha decay
Slide 11
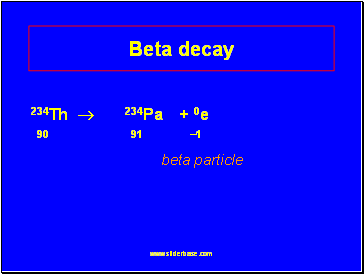
Beta decay
234Th ® 234Pa + 0e
90 91 1
beta particle
Slide 12
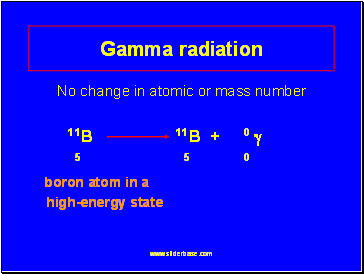
Gamma radiation
No change in atomic or mass number
11B 11B + 0
5 5 0
boron atom in a
high-energy state
Slide 13
Contents
- Ch 9 - Nuclear Radiation
- Nuclear Emissions
- Nuclear Equations
- Producing Radioactive Isotopes
- Half-Life of a Radioisotope
- Fission VS Fusion
- Uses & Effects
Last added presentations
- Ch 9 Nuclear Radiation
- Motion
- Practical Applications of Solar Energy
- Simulation at NASA for the Space Radiation Effort
- Radiation Safety and Operations
- Static and Kinetic Friction
- Mechanics Lecture