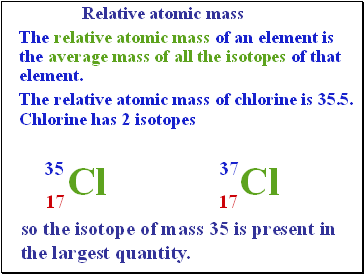Atoms and the Periodic tablePage
2
2
Slide 9
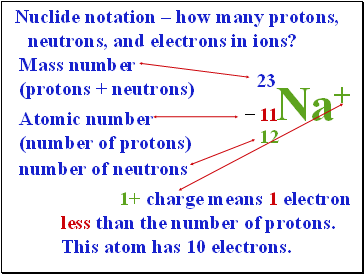
Nuclide notation – how many protons, neutrons, and electrons in ions?
23
Mass number
(protons + neutrons)
Na+
11
Atomic number
(number of protons)
–
12
number of neutrons
1+ charge means 1 electron less than the number of protons. This atom has 10 electrons.
Slide 10
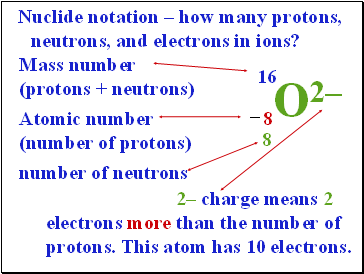
Nuclide notation – how many protons, neutrons, and electrons in ions?
16
Mass number
(protons + neutrons)
O2–
8
Atomic number
(number of protons)
–
8
number of neutrons
2– charge means 2 electrons more than the number of protons. This atom has 10 electrons.
Slide 11
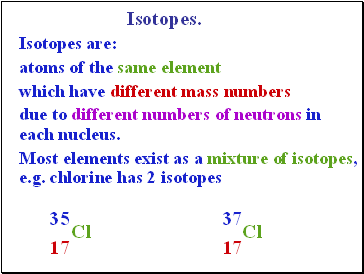
Isotopes.
Isotopes are:
atoms of the same element
which have different mass numbers
due to different numbers of neutrons in each nucleus.
Most elements exist as a mixture of isotopes, e.g. chlorine has 2 isotopes
35
Cl
17
37
Cl
17
Slide 12
Relative atomic mass
The relative atomic mass of an element is the average mass of all the isotopes of that element.
The relative atomic mass of chlorine is 35.5. Chlorine has 2 isotopes
35
Cl
17
37
Cl
17
so the isotope of mass 35 is present in the largest quantity.
1 2
Contents
- Atoms and the Periodic Table.
- The Periodic Table of the Elements.
- Groups of elements have names
- Isotopes.
- Relative atomic mass
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Magnetic field uses sound waves to ignite sun's ring of fire
- Mechanics Lecture
- Solar Energy
- Resource Acquisition and Transport in Vascular Plants
- The Effects of Radiation on Living Things