Spectral linesPage
1
1
Slide 1
Spectral Lines
Celestial Fingerprinting
Slide 2
Goals
From light we learn about
Composition
Motion
Slide 3
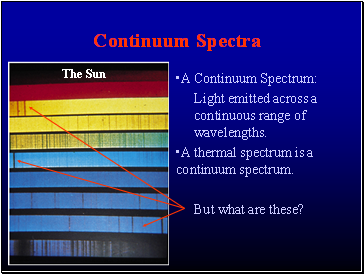
Continuum Spectra
A Continuum Spectrum:
Light emitted across a continuous range of wavelengths.
A thermal spectrum is a continuum spectrum.
But what are these?
The Sun
Slide 4
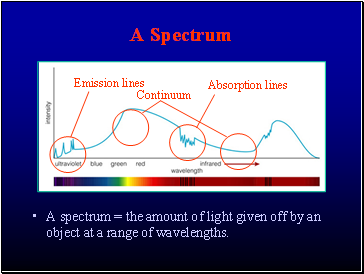
A Spectrum
A spectrum = the amount of light given off by an object at a range of wavelengths.
Slide 5
Continuum Concept Test
The sun shines on a cold airless asteroid made of black coal. What light from the asteroid do we detect?
No light at all.
Some reflected visible light.
Some reflected visible, plus emitted visible light.
Some reflected visible, plus emitted infrared light.
Some reflected visible, plus emitted visible and emitted infrared light.
Slide 6
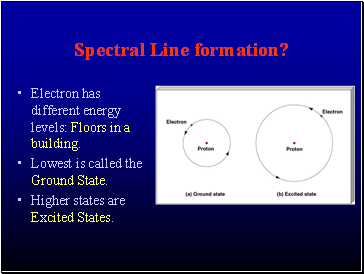
Spectral Line formation?
Electron has different energy levels: Floors in a building.
Lowest is called the Ground State.
Higher states are Excited States.
Slide 7
Changing Levels
If you add the RIGHT amount of energy to an atom, the electron will jump up energy floors.
If the electron drops down energy floors, the atom gives up the same amount energy.
From before, LIGHT IS ENERGY: E = hc/l
Slide 8
Kirchhoff’s Laws
Light of all wavelengths shines on an atom.
Only light of an energy equal to the difference between “floors” will be absorbed and cause electrons to jump up in floors.
The rest of the light passes on by to our detector.
We see an absorption spectrum: light at all wavelengths minus those specific wavelengths.
Slide 9
Absorption Lines
Pass light at all wavelengths through low density gas.
Pass this light through our spectrometer.
We see the continuum spectrum.
Now it’s MISSING certain wavelengths.
Slide 10
Absorption
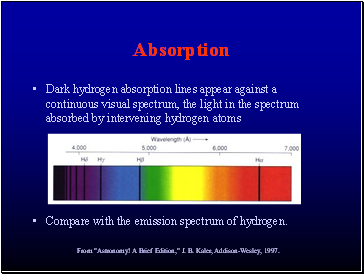
Dark hydrogen absorption lines appear against a continuous visual spectrum, the light in the spectrum absorbed by intervening hydrogen atoms
Compare with the emission spectrum of hydrogen.
Contents
- Spectral Lines
- Goals
- Continuum Spectra
- A Spectrum
- Continuum Concept Test
- Spectral Line formation?
- Changing Levels
- Kirchhoff’s Laws
- Absorption Lines
- Absorption
- Kirchhoff’s Laws Cont…
- Spectral Lines
- Emission Lines
- Multiple elements
- Different stars, different spectra
- To Sum Up…
- Concept Test
- The Sun
- Helium
- Doppler Shift
- Concept Test
Last added presentations
- Mechanics Lecture
- Health Physics
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Sound
- Soil and Plant Nutrition
- Sensory and Motor Mechanisms
- Radioactivity and Nuclear Reactions