Atomic Structure IPage
2
2
Light is a wave…right?
Einstein’s interpretation of the photoelectric effect (1905) was that light is quantized in packets of set energy called photons. (He won the Nobel Prize for this.)
This meant that light had characteristics of particles!
Slide 13
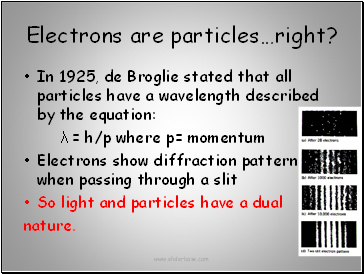
Electrons are particles…right?
In 1925, de Broglie stated that all particles have a wavelength described by the equation:
λ = h/p where p= momentum
Electrons show diffraction pattern when passing through a slit
So light and particles have a dual
nature.
Slide 14
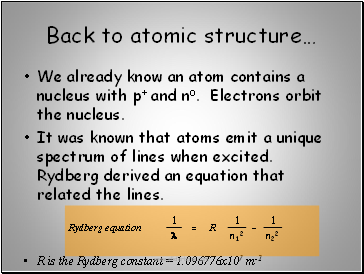
Back to atomic structure…
We already know an atom contains a nucleus with p+ and no. Electrons orbit the nucleus.
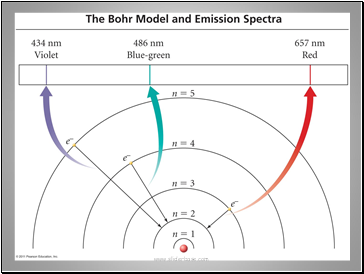
It was known that atoms emit a unique spectrum of lines when excited. Rydberg derived an equation that related the lines.
R is the Rydberg constant = 1.096776x107 m-1
Slide 15
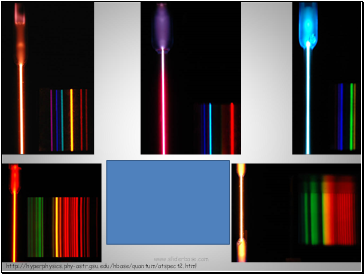
Flame test colors derive from electrons changing energy levels.
Slide 16
http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/atspect2.html
Atomic emission spectra
Clockwise from lower left:
neon, helium, hydrogen,
mercury, nitrogen
Slide 17
Slide 18
Slide 19
Electron locations
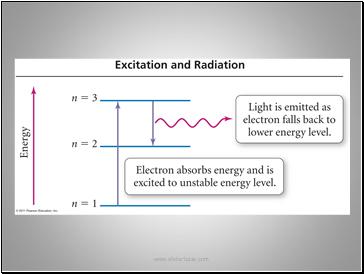
When an electron occupies its usual energy level it is in the ground state.
When an electron absorbs a photon and moves to a higher energy level it is in an excited state.
The energy levels are “quantized”. Atoms can only transition between set levels.
Why are the levels set where they are?
Slide 20
More on electrons as waves
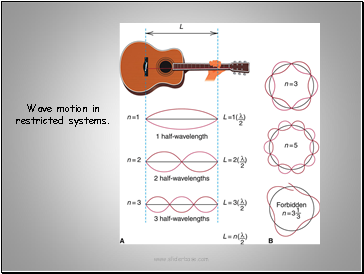
Since electrons have wave motion Schrödinger applied the classic wave equations to the motion of a hydrogen electron. Certain wavelengths reinforced each other and were allowed.
This generated regions occupied by an electron of set energy termed orbitals.
Slide 21
Wave motion in restricted systems.
Slide 22
More on electrons as waves
Heisenberg stated that in measuring the electron there is uncertainty so we can only calculate a probable location for the electron. This is called the Heisenberg Uncertainty Principle.
Contents
- Atomic Structure
- Particle or Wave?
- Light is a wave…right?
- Electrons are particles…right?
- Back to atomic structure…
- Electron locations
- More on electrons as waves
Last added presentations
- History of Modern Astronomy
- Geophysical Concepts, Applications and Limitations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Thermal Energy
- Buoyancy
- Friction
- Gravitation