Percent Composition, Empirical and Molecular FormulasPage
2
2
1. Find the formula mass of C3H5O2
3(12.01 g) + 5(1.01) + 2(16.00) = 73.08 g
Slide 11
Finding the Molecular Formula
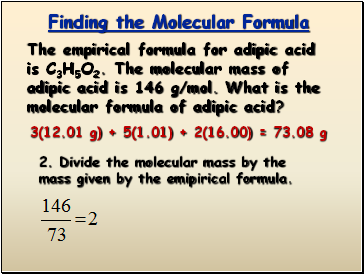
The empirical formula for adipic acid is C3H5O2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid?
3(12.01 g) + 5(1.01) + 2(16.00) = 73.08 g
2. Divide the molecular mass by the mass given by the emipirical formula.
Slide 12
Finding the Molecular Formula
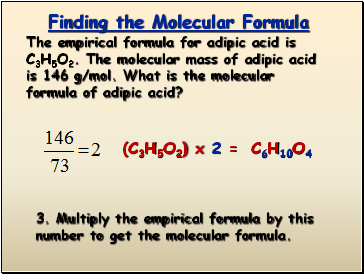
The empirical formula for adipic acid is C3H5O2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid?
3. Multiply the empirical formula by this number to get the molecular formula.
(C3H5O2) x 2 =
C6H10O4
Go to page:
1 2
1 2
Contents
- Calculating Percentage Composition
- Formulas
- Empirical Formula Determination
- Finding the Molecular Formula
Last added presentations
- Static and Kinetic Friction
- Newton's Laws
- Buoyancy
- Upcoming Classes
- Radioactivity and Nuclear Reactions
- Radiation
- Health Physics
© 2010-2025 powerpoint presentations