PeriodicTable - GamePage
1
1
Slide 1
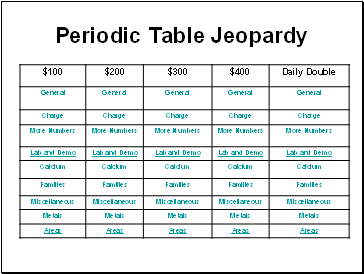
Periodic Table Jeopardy
Slide 2
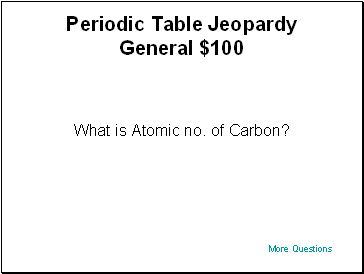
Periodic Table Jeopardy General $100
What is Atomic no. of Carbon?
More Questions
Slide 3
General
Periodic Table Jeopardy $200
How many protons are in Oxygen?
Slide 4
Periodic Table Jeopardy General $300
What is the mass number of Carbon ?
More Questions
Slide 5
Periodic Table Jeopardy General $400
What is the atomic mass of Boron?
More Questions
Slide 6
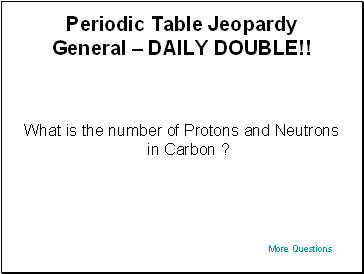
Periodic Table Jeopardy General – DAILY DOUBLE!!
What is the number of Protons and Neutrons in Carbon ?
More Questions
Slide 7
Charge
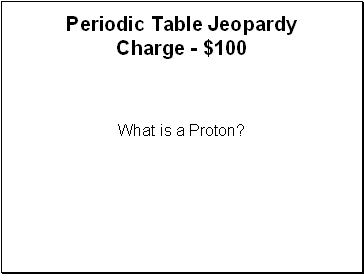
Periodic Table Jeopardy - $100
What is a Proton?
Slide 8
Periodic Table Jeopardy Charge - $200
What is a Electron?
More Questions
Slide 9
Periodic Table Jeopardy Charge - $300
What is a Neutron?
Slide 10
Periodic Table Jeopardy Charge - $400
Which Atom gains 1 electron?
More Questions
Slide 11
Periodic Table Jeopardy Charge – DAILY DOUBLE!!
Which atom loses 2 electrons?
More Questions
Slide 12
More Numbers
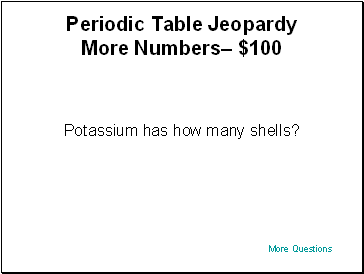
Periodic Table Jeopardy– $100
Potassium has how many shells?
More Questions
Slide 13
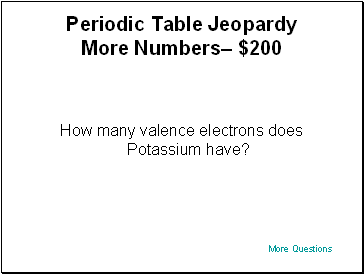
Periodic Table Jeopardy More Numbers– $200
How many valence electrons does Potassium have?
More Questions
Slide 14
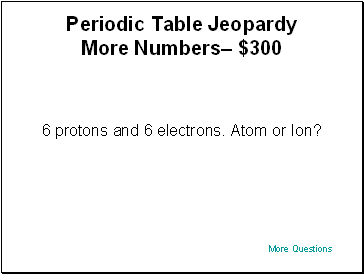
Periodic Table Jeopardy More Numbers– $300
6 protons and 6 electrons. Atom or Ion?
More Questions
Slide 15
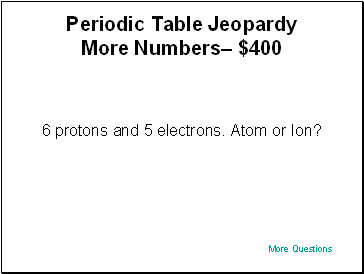
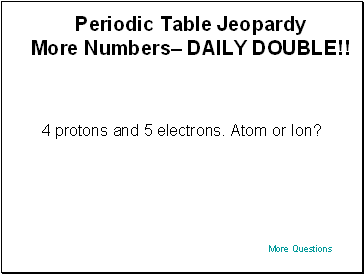
Periodic Table Jeopardy More Numbers– $400
6 protons and 5 electrons. Atom or Ion?
More Questions
Slide 16
Contents
Last added presentations
- Sensory and Motor Mechanisms
- Friction
- Soil and Plant Nutrition
- Newton's laws of motion
- Motion
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Understanding Heat Transfer, Conduction, Convection and Radiation