Properties of GasesPage
1
1
Slide 1
Gases
Slide 2
The Nature of Gases
Gases expand to fill their containers
Gases are fluid – they flow
Gases have low density
1/1000 the density of the equivalent liquid or solid
Gases are compressible
Gases effuse and diffuse
Slide 3
Ideal Gases
Ideal gases are imaginary gases that perfectly
fit all of the assumptions of the kinetic molecular theory.
Gases consist of tiny particles that are
far apart relative to their size.
Collisions between gas particles and between
particles and the walls of the container are
elastic collisions
No kinetic energy is lost in elastic
collisions
Slide 4
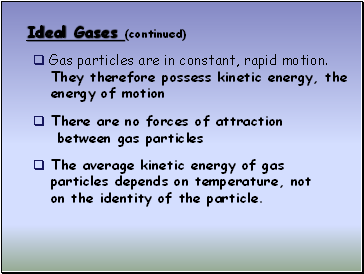
Ideal Gases (continued)
Gas particles are in constant, rapid motion.
They therefore possess kinetic energy, the
energy of motion
There are no forces of attraction
between gas particles
The average kinetic energy of gas
particles depends on temperature, not
on the identity of the particle.
Slide 5
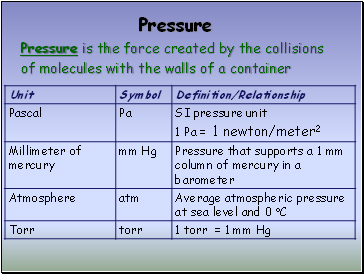
Pressure
Pressure is the force created by the collisions
of molecules with the walls of a container
Slide 6
Standard Pressure
1 standard atmosphere (atm)
101.3 kPa (kilopascals)
14.7 lbs/in2
760 mm Hg (millimeters of mercury)
760 torr
Slide 7
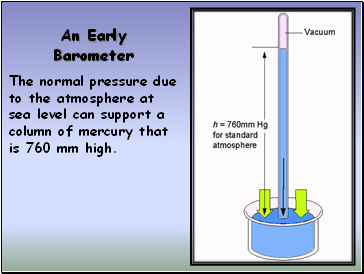
Measuring Pressure
The first device for measuring atmospheric
pressure was developed by Evangelista Torricelli
during the 17th century.
The device was called a “barometer”
Baro = weight
Meter = measure
Slide 8
An Early Barometer
The normal pressure due to the atmosphere at sea level can support a column of mercury that is 760 mm high.
Slide 9
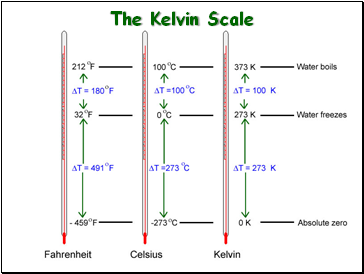
The Kelvin Scale
Slide 10
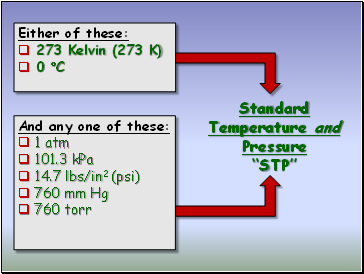
Standard Temperature and Pressure “STP”
Either of these:
273 Kelvin (273 K)
0 C
And any one of these:
1 atm
101.3 kPa
14.7 lbs/in2 (psi)
760 mm Hg
760 torr
Contents
- The Nature of Gases
- Ideal Gases
- Pressure
- Standard Pressure
- Measuring Pressure
- An Early Barometer
- The Kelvin Scale
Last added presentations
- Simulation at NASA for the Space Radiation Effort
- Upcoming Classes
- Motion
- Practical Applications of Solar Energy
- Sensory and Motor Mechanisms
- Solar Energy
- Resource Acquisition and Transport in Vascular Plants