Thermochemical CalculationsPage
1
1
Slide 1
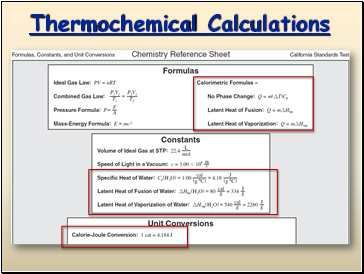
Thermochemical Calculations
Slide 2
CA Standards
Slide 3
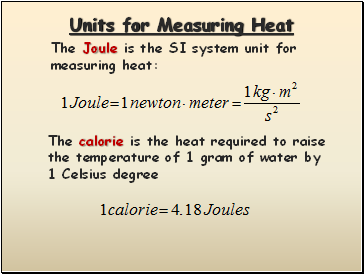
Units for Measuring Heat
The Joule is the SI system unit for measuring heat:
The calorie is the heat required to raise the temperature of 1 gram of water by 1 Celsius degree
Slide 4
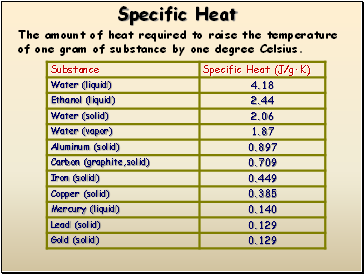
Specific Heat
The amount of heat required to raise the temperature of one gram of substance by one degree Celsius.
Slide 5
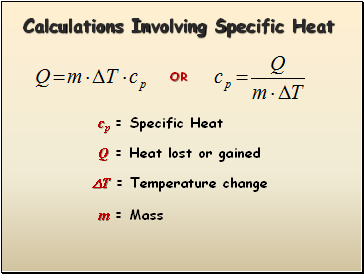
Calculations Involving Specific Heat
cp = Specific Heat
Q = Heat lost or gained
T = Temperature change
OR
m = Mass
Slide 6
Specific Heat
The amount of heat required to raise the temperature of one gram of substance by one degree Celsius.
Slide 7
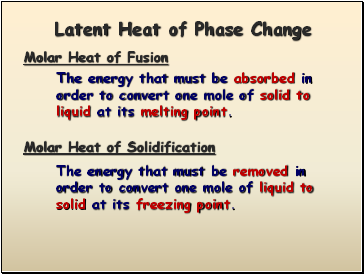
Latent Heat of Phase Change
Molar Heat of Fusion
The energy that must be absorbed in order to convert one mole of solid to liquid at its melting point.
The energy that must be removed in order to convert one mole of liquid to solid at its freezing point.
Molar Heat of Solidification
Slide 8
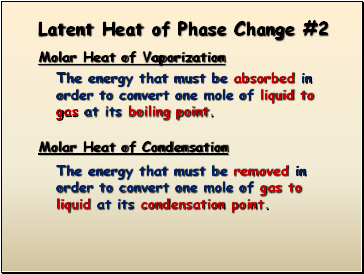
Latent Heat of Phase Change #2
Molar Heat of Vaporization
The energy that must be absorbed in order to convert one mole of liquid to gas at its boiling point.
The energy that must be removed in order to convert one mole of gas to liquid at its condensation point.
Molar Heat of Condensation
Slide 9
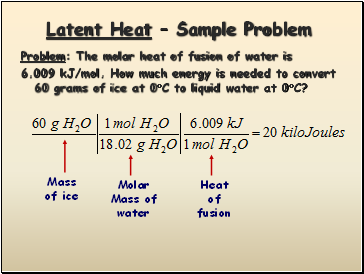
Latent Heat – Sample Problem
Problem: The molar heat of fusion of water is
6.009 kJ/mol. How much energy is needed to convert 60 grams of ice at 0C to liquid water at 0C?
Mass
of ice
Molar
Mass of
water
Heat
of
fusion
Slide 10
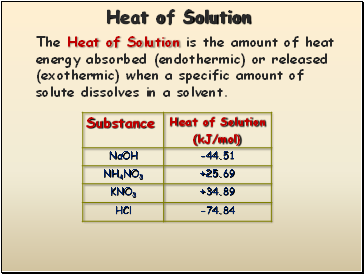
Heat of Solution
The Heat of Solution is the amount of heat energy absorbed (endothermic) or released (exothermic) when a specific amount of solute dissolves in a solvent.
Contents
- Thermochemical Calculations
- Units for Measuring Heat
- Specific Heat
- Calculations Involving Specific Heat
- Specific Heat
- Latent Heat of Phase Change
- Latent Heat – Sample Problem
- Heat of Solution
Last added presentations
- Newton’s laws of motion
- Newton’s law of universal gravitation
- Simulation at NASA for the Space Radiation Effort
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Newton’s third law of motion
- Solar Energy
- Sensory and Motor Mechanisms