Balancing Chemical equations (Balancing Chemical Equations)Page
1
1
Slide 1
Algebraic Solving Method
Balancing Chemical Equations
Slide 2
Balance This Equation
Pb(N3)2 + Cr(MnO4)2 Cr2O3+ MnO2 + Pb3O4 + NO
Slide 3
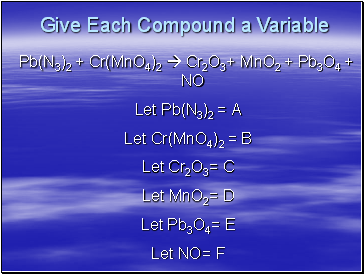
Give Each Compound a Variable
Pb(N3)2 + Cr(MnO4)2 Cr2O3+ MnO2 + Pb3O4 + NO
Let Pb(N3)2 = A
Let Cr(MnO4)2 = B
Let Cr2O3= C
Let MnO2= D
Let Pb3O4= E
Let NO= F
Slide 4
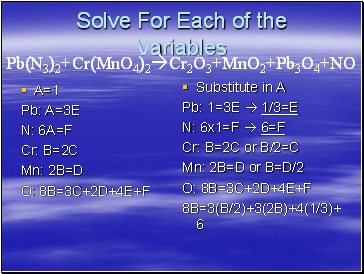
Solve For Each of the Variables
Pb(N3)2+Cr(MnO4)2Cr2O3+MnO2+Pb3O4+NO
Let A=1
Pb: A=3E
* A is the amount of Pb on the left side of the equation
*3E is the amount on the left side
Slide 5
Solve For Each of the Variables
A=1
Pb: A=3E
N: 6A=F
Cr: B=2C
Mn: 2B=D
O: 8B=3C+2D+4E+F
Substitute in A
Pb: 1=3E 1/3=E
N: 6x1=F 6=F
Cr: B=2C or B/2=C
Mn: 2B=D or B=D/2
O: 8B=3C+2D+4E+F
8B=3(B/2)+3(2B)+4(1/3)+6
Pb(N3)2+Cr(MnO4)2Cr2O3+MnO2+Pb3O4+NO
Slide 6
Solve for Oxygen
O: 8B=3B/2+4B+4/3+6
Find a lowest common multiple of 2 and 3
Multiply each side by 6
[6x] 8B=(3B/2)+(4B+4/3)+6
48B=9B+24B+8+36
48B=33B+44
15B=44
Slide 7
Simplify Your Fractions
A=1
B=44/15
C=(B/2)x(44/15)
C=44/30
D=2B
D=(2/1)x(44/15)
D=88/15
E=1/3
F=6
Find a GCD and multiply each.
A=15
B=44
C=22
D=88
E=5
F=90
Slide 8
Now You Have A Finished Equation
Substitute each value into the equation.
A=15 D=88
B=44 E=5
C=22 F=90
15Pb(N3)2 +44 Cr(MnO4)2 22Cr2O3+ 88MnO2 + 5Pb3O4 +90 NO
Contents
- Algebraic Solving Method
- Balance This Equation
- Give Each Compound a Variable
- Solve For Each of the Variables
- Solve for Oxygen
- Simplify Your Fractions
- Now You Have A Finished Equation
Last added presentations
- Motion
- The Effects of Radiation on Living Things
- Sound
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Newton’s Law of Gravity
- Radiation Safety and Operations
- Space Radiation