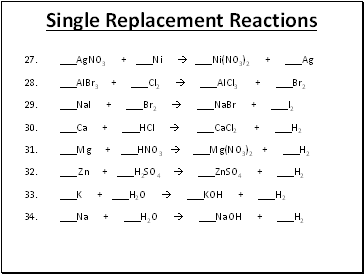Balancing Chemical EquationsPage
1
1
Slide 1
Balancing Chemical Equations
Slide 2
CA Standards
Students know how to describe chemical reactions by writing balanced equations.
Slide 3
Law of Conservation of Mass
In ordinary chemical reactions, the total mass of reacting substances is equal to the total mass of products
All atoms on the reactant side must appear on the product side, and in equal numbers
No new elements may appear
No elements may disappear
Slide 4
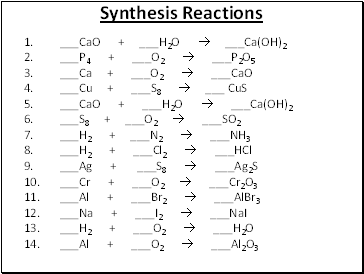
Synthesis Reactions
1. _CaO + _H2O _Ca(OH)2
2. _P4 + _O2 _P2O5
3. _Ca + _O2 _CaO
4. _Cu + _S8 _ CuS
5. _CaO + _H2O _Ca(OH)2
6. _S8 + _O2 _SO2
7. _H2 + _N2 _NH3
8. _H2 + _Cl2 _HCl
9. _Ag + _S8 _Ag2S
10. _Cr + _O2 _Cr2O3
11. _Al + _Br2 _AlBr3
12. _Na + _I2 _NaI
13. _H2 + _O2 _H2O
14. _Al + _O2 _Al2O3
Slide 5
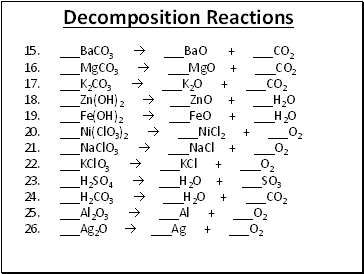
Decomposition Reactions
15. _BaCO3 _BaO + _CO2
16. _MgCO3 _MgO + _CO2
17. _K2CO3 _K2O + _CO2
18. _Zn(OH)2 _ZnO + _H2O
19. _Fe(OH)2 _FeO + _H2O
20. _Ni(ClO3)2 _NiCl2 + _O2
21. _NaClO3 _NaCl + _O2
22. _KClO3 _KCl + _O2
23. _H2SO4 _H2O + _SO3
24. _H2CO3 _H2O + _CO2
25. _Al2O3 _Al + _O2
26. _Ag2O _Ag + _O2
Slide 6
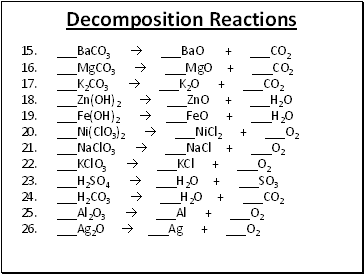
Decomposition Reactions
15. _BaCO3 _BaO + _CO2
16. _MgCO3 _MgO + _CO2
17. _K2CO3 _K2O + _CO2
18. _Zn(OH)2 _ZnO + _H2O
19. _Fe(OH)2 _FeO + _H2O
20. _Ni(ClO3)2 _NiCl2 + _O2
21. _NaClO3 _NaCl + _O2
22. _KClO3 _KCl + _O2
23. _H2SO4 _H2O + _SO3
24. _H2CO3 _H2O + _CO2
25. _Al2O3 _Al + _O2
26. _Ag2O _Ag + _O2
Slide 7
Single Replacement Reactions
27. _AgNO3 + _Ni _Ni(NO3)2 + _Ag
28. _AlBr3 + _Cl2 _AlCl3 + _Br2
29. _NaI + _Br2 _NaBr + _I2
30. _Ca + _HCl _CaCl2 + _H2
31. _Mg + _HNO3 _Mg(NO3)2 + _H2
32. _ Zn + _H2SO4 _ZnSO4 + _H2
33. _K + _H2O _KOH + _H2
34. _Na + _H2O _NaOH + _H2
Slide 8
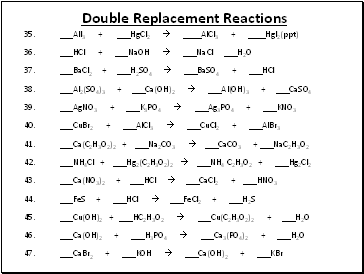
Double Replacement Reactions
1 2
Contents
- Balancing Chemical Equations
- Law of Conservation of Mass
- Synthesis Reactions
- Decomposition Reactions
- Single Replacement Reactions
- Double Replacement Reactions
- Combustion Reactions
Last added presentations
- The Effects of Radiation on Living Things
- Resource Acquisition and Transport in Vascular Plants
- Radiation Safety and Operations
- Buoyancy
- History of Modern Astronomy
- Ch 9 Nuclear Radiation
- Static and Kinetic Friction