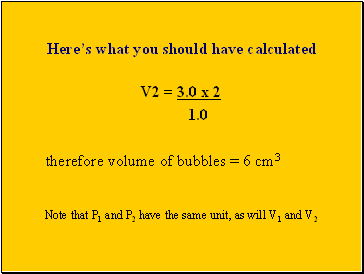Boyle's LawPage
2
2
Curved lines are hard to recognise, so we plot the volume against the reciprocal of pressure (ie. 1/p)
This time the points lie close to a straight line through the origin.
This means volume is directly proportional to 1/pressure or
volume is inversely proportional to pressure
Slide 11
This leads us back to Boyle’s Law
Boyle’s Law: for a fixed mass of gas kept at constant temperature the volume of the gas is inversely proportional to its pressure.
Slide 12
Problem
A deep sea diver is working at a depth where the pressure is 3.0 atmospheres. He is breathing out air bubbles. The volume of each air bubble is 2 cm2. At the surface the pressure is 1 atmosphere. What is the volume of each bubble when it reaches the surface?
Slide 13
How we work this out
We assume that the temperature is constant, so Boyle’s Law applies:
Formula first: P1 x V1 = P2 x V2
Then numbers:= 1.0 x 2 = 3.0 x V2
Now rearrange the numbers so that you have V2 on one side, and the rest of the numbers on the other side of the ‘equals’ symbol.
Slide 14
Here’s what you should have calculated
V2 = 3.0 x 2
1.0
therefore volume of bubbles = 6 cm3
Note that P1 and P2 have the same unit, as will V1 and V2
1 2
Contents
- What is Boyle’s Law?
- How can we write Boyle’s Law as a formula?
- How can we investigate Boyle’s Law?
- Boyle’s Law apparatus
- Below are some results of an experiment
- What these experimental results show
- Another way of plotting the data
- This leads us back to Boyle’s Law
- Problem
- How we work this out
Last added presentations
- Sensory and Motor Mechanisms
- Newton’s Laws of Motion
- Geophysical Concepts, Applications and Limitations
- Health Physics
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Newton’s third law of motion
- Gravitation