Osmosis & DiffusionPage
2
2
Isotonic Solution - both solutions have same concentrations of solute.
Slide 13
Plant and Animal Cells put into
various solutions
Slide 14
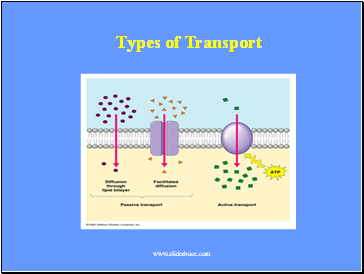
Types of Transport
Slide 15
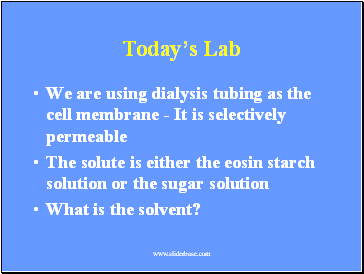
Today’s Lab
We are using dialysis tubing as the cell membrane - It is selectively permeable
The solute is either the eosin starch solution or the sugar solution
What is the solvent?
Slide 16
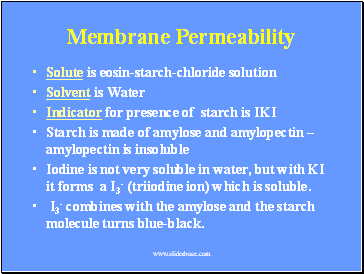
Membrane Permeability
Solute is eosin-starch-chloride solution
Solvent is Water
Indicator for presence of starch is IKI
Starch is made of amylose and amylopectin – amylopectin is insoluble
Iodine is not very soluble in water, but with KI it forms a I3- (triiodine ion) which is soluble.
I3- combines with the amylose and the starch molecule turns blue-black.
Slide 17
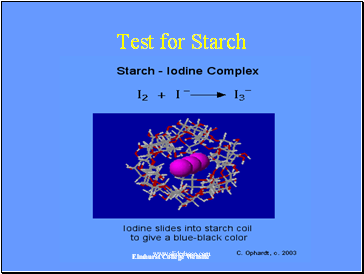
Test for Starch
Elmhurst College Website
Slide 18
Test for Chloride ions
Indicator for presence of chloride ions is silver nitrate, AgNO3
A white precipitate, AgCl, forms if chloride is present.
Slide 19
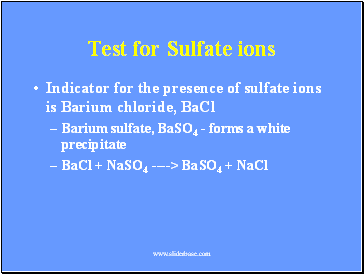
Test for Sulfate ions
Indicator for the presence of sulfate ions is Barium chloride, BaCl
Barium sulfate, BaSO4 - forms a white precipitate
BaCl + NaSO4 ----> BaSO4 + NaCl
Slide 20
Living Cells
Beet cubes will be used to see the result of boiling and adding alcohol to a live membrane.
Yeast cells are used to see effects of heat
Fern gametophytes are used to see result of putting live cells in solutions of varying tonicity
Slide 21
Osmosis
We will make an osmometer to see osmosis
Sugar solution in a dialysis tube is used to simulate a cell membrane.
Various concentrations of solute may be used around the room.
1 2
Contents
- Cell Membranes
- Functions of Membranes
- Phospholipid Bilayer
- Fluid Mosaic Model
- Blood-Brain Barrier
- Solutions
- Methods of Transport Across Membranes
- Methods of Transport Across Membranes
- Diffusion
- Tonicity is a relative term
- Types of Transport
- Today’s Lab
- Membrane Permeability
- Test for Starch
- Test for Chloride ions
- Test for Sulfate ions
- Living Cells
Last added presentations
- Newton's laws of motion
- Health Physics
- Newton’s third law of motion
- Newton’s Law of Gravity
- Sound
- Simulation at NASA for the Space Radiation Effort
- Upcoming Classes