Properties of WaterPage
2
2
Blood & Cytoplasm are suspensions
Slide 12
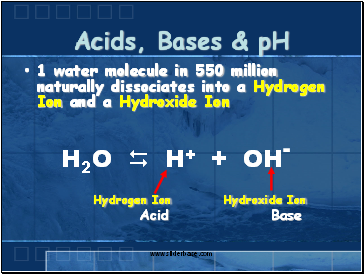
Acids, Bases & pH
1 water molecule in 550 million naturally dissociates into a Hydrogen Ion and a Hydroxide Ion
Hydrogen Ion Hydroxide Ion
Acid Base
H2O H+ + OH-
Slide 13
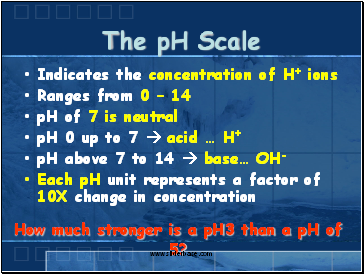
The pH Scale
Indicates the concentration of H+ ions
Ranges from 0 – 14
pH of 7 is neutral
pH 0 up to 7 acid … H+
pH above 7 to 14 base… OH-
Each pH unit represents a factor of 10X change in concentration
How much stronger is a pH3 than a pH of 5?
Slide 14
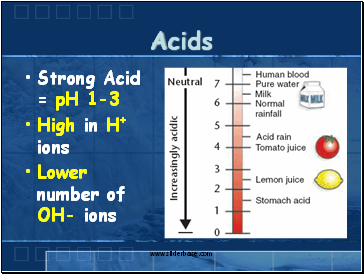
Acids
Strong Acid = pH 1-3
High in H+ ions
Lower number of OH- ions
Slide 15
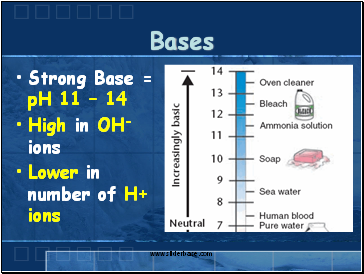
Bases
Strong Base = pH 11 – 14
High in OH-ions
Lower in number of H+ ions
Slide 16
Buffers
Weak acids or bases that react with strong acids or bases
Made by the body
Prevent sharp, sudden changes in pH (keep pH neutral)
Weak Acid
Weak Base
Go to page:
1 2
1 2
Contents
- The Water Molecule
- Hydrogen Bonds
- Cohesion
- Adhesion & Capillarity
- Solutions & Suspensions
- Properties of Solutions
- Ionic Solutions
- Suspensions
- Acids, Bases & pH
- The pH Scale
- Acids
- Bases
- Buffers
Last added presentations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Newton’s laws of motion
- Geophysical Concepts, Applications and Limitations
- Ch 9 Nuclear Radiation
- Motion
- The Effects of Radiation on Living Things
© 2010-2025 powerpoint presentations