Atomic Structure TimelinePage
1
1
Slide 1
Atomic Structure Timeline
Use the following information to complete the lecture handout.
On tomorrow’s quiz, you will be expected to…
draw the atomic models
match scientists to their experiments and discoveries
place the models in chronological order
Slide 2
Democritus (400 B.C.)
Proposed that matter was composed of tiny indivisible particles
Not based on experimental data
Greek: atomos
Slide 3
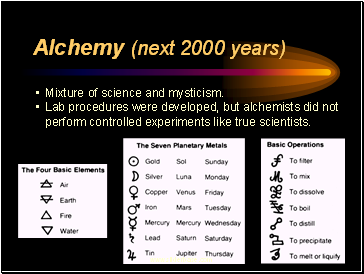
Alchemy (next 2000 years)
Mixture of science and mysticism.
Lab procedures were developed, but alchemists did not perform controlled experiments like true scientists.
Slide 4
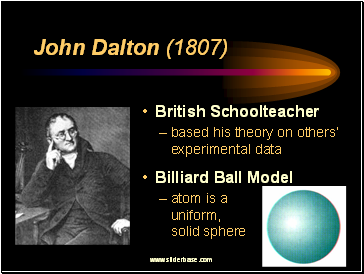
John Dalton (1807)
British Schoolteacher
based his theory on others’ experimental data
Billiard Ball Model
atom is a uniform, solid sphere
Slide 5
John Dalton
Dalton’s Four Postulates
1. Elements are composed of small indivisible particles called atoms.
2. Atoms of the same element are identical. Atoms of different elements are different.
3. Atoms of different elements combine together in simple proportions to create a compound.
4. In a chemical reaction, atoms are rearranged, but not changed.
Slide 6
Henri Becquerel (1896)
Discovered radioactivity
spontaneous emission of radiation from the nucleus
Three types:
alpha () - positive
beta () - negative
gamma () - neutral
Slide 7
J. J. Thomson (1903)
Cathode Ray Tube Experiments
beam of negative particles
Discovered Electrons
negative particles within the atom
Plum-pudding Model
Slide 8
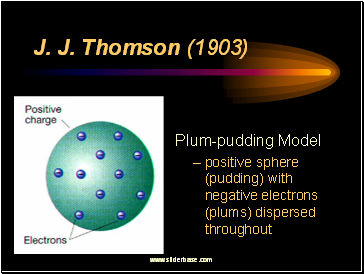
J. J. Thomson (1903)
Plum-pudding Model
positive sphere (pudding) with negative electrons (plums) dispersed throughout
Slide 9
Ernest Rutherford (1911)
Gold Foil Experiment
Discovered the nucleus
dense, positive charge in the center of the atom
Nuclear Model
Slide 10
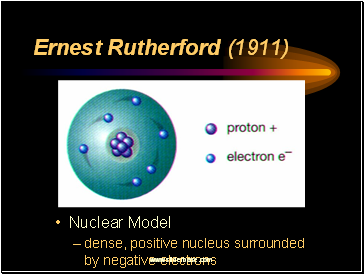
Ernest Rutherford (1911)
Nuclear Model
dense, positive nucleus surrounded by negative electrons
Slide 11
1 2
Contents
- Atomic Structure Timeline
- Democritus (400 B.C.)
- Alchemy (next 2000 years)
- John Dalton (1807)
- Henri Becquerel (1896)
- J. J. Thomson (1903)
- Ernest Rutherford (1911)
- Niels Bohr (1913)
- Erwin Schrödinger (1926)
- James Chadwick (1932)
Last added presentations
- Practical Applications of Solar Energy
- Madame Marie Curie
- Friction
- Resource Acquisition and Transport in Vascular Plants
- The Effects of Radiation on Living Things
- Motion
- Newton’s Law of Gravity