Atomic Structure TimelinePage
2
2
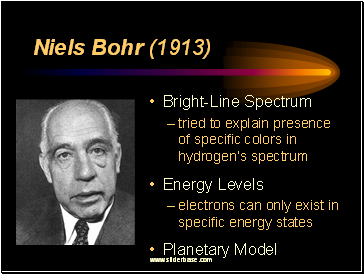
Niels Bohr (1913)
Bright-Line Spectrum
tried to explain presence of specific colors in hydrogen’s spectrum
Energy Levels
electrons can only exist in specific energy states
Planetary Model
Slide 12
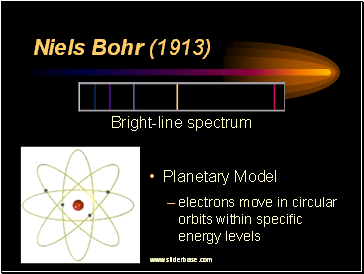
Niels Bohr (1913)
Planetary Model
electrons move in circular orbits within specific energy levels
Bright-line spectrum
Slide 13
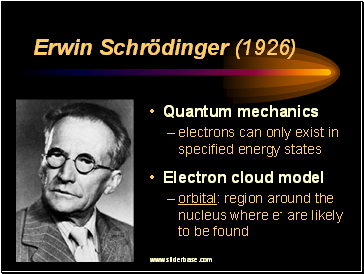
Erwin Schrödinger (1926)
Quantum mechanics
electrons can only exist in specified energy states
Electron cloud model
orbital: region around the nucleus where e- are likely to be found
Slide 14
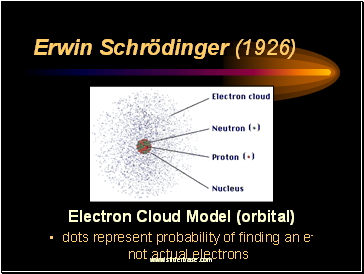
Erwin Schrödinger (1926)
Electron Cloud Model (orbital)
dots represent probability of finding an e- not actual electrons
Slide 15
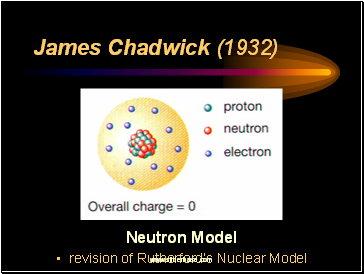
James Chadwick (1932)
Discovered neutrons
neutral particles in the nucleus of an atom
Joliot-Curie Experiments
based his theory on their experimental evidence
Slide 16
James Chadwick (1932)
Neutron Model
revision of Rutherford’s Nuclear Model
1 2
Contents
- Atomic Structure Timeline
- Democritus (400 B.C.)
- Alchemy (next 2000 years)
- John Dalton (1807)
- Henri Becquerel (1896)
- J. J. Thomson (1903)
- Ernest Rutherford (1911)
- Niels Bohr (1913)
- Erwin Schrödinger (1926)
- James Chadwick (1932)
Last added presentations
- Upcoming Classes
- Waves & Sound
- Radiation Safety and Operations
- Heat-Energy on the Move
- Motion
- Newton’s third law of motion
- Newton’s Laws of Motion