Hess's LawPage
1
1
Slide 1
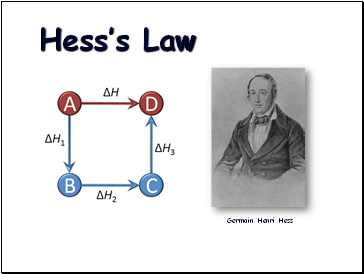
Hessís Law
Germain Henri Hess
Slide 2
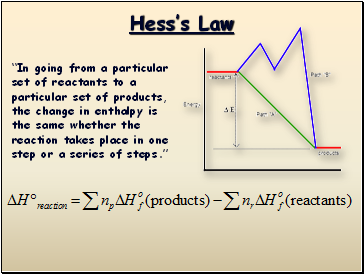
Hessís Law
ďIn going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or a series of steps.Ē
Slide 3
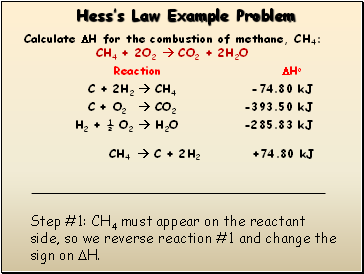
Hessís Law Example Problem
Step #1: CH4 must appear on the reactant side, so we reverse reaction #1 and change the sign on H.
CH4 C + 2H2 +74.80 kJ
Slide 4
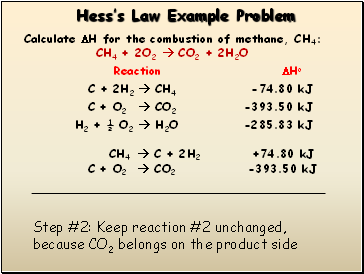
Hessís Law Example Problem
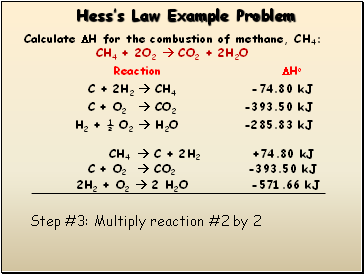
CH4 C + 2H2 +74.80 kJ
Step #2: Keep reaction #2 unchanged, because CO2 belongs on the product side
C + O2 CO2 -393.50 kJ
Slide 5
Hessís Law Example Problem
CH4 C + 2H2 +74.80 kJ
C + O2 CO2 -393.50 kJ
Step #3: Multiply reaction #2 by 2
2H2 + O2 2 H2O -571.66 kJ
Slide 6
Hessís Law Example Problem
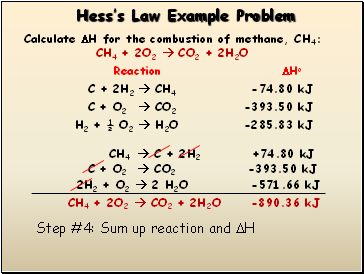
CH4 C + 2H2 +74.80 kJ
C + O2 CO2 -393.50 kJ
2H2 + O2 2 H2O -571.66 kJ
Step #4: Sum up reaction and H
CH4 + 2O2 CO2 + 2H2O
-890.36 kJ
Slide 7
Calculation of Heat of Reaction
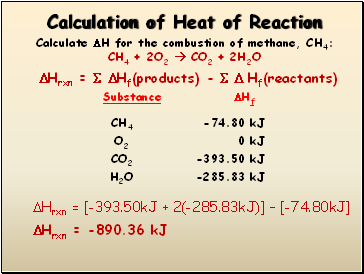
Hrxn = Hf(products) - Hf(reactants)
Hrxn = [-393.50kJ + 2(-285.83kJ)] Ė [-74.80kJ]
Hrxn = -890.36 kJ
Contents
Last added presentations
- Friction
- Resource Acquisition and Transport in Vascular Plants
- Newton's laws of motion
- Newtonís laws of motion
- Motion
- Ch 9 Nuclear Radiation
- History of Modern Astronomy