Thermal EnergyPage
1
1
Slide 1
Heat and Internal Energy
Internal Energy U is the total energy associated with the microscopic components of the system
Includes kinetic and potential energy associated with the random translational, rotational and vibrational motion of the atoms or molecules
Also includes the intermolecular potential energy
Does not include macroscopic kinetic energy or external potential energy
Heat refers to the transfer of energy between a system and its environment due to a temperature difference between them
Amount of energy transferred by heat designated by symbol Q
A system does not have heat, just like it does not have work (heat and work speak to transfer of energy)
Slide 2
Units of Heat
The historical unit of heat was the calorie
A calorie is the amount of energy necessary to raise the temperature of 1 g of water from 14.5°C to 15.5°C
A Calorie (food calorie, with a capital C) is 1000 cal
Since heat (like work) is a measure of energy transfer, its SI unit is the joule
1 cal = 4.186 J (“Mechanical Equivalent of Heat”)
New definition of the calorie
The unit of heat in the U.S. customary system is the British thermal unit (BTU)
Defined as the amount of energy necessary to raise the temperature of 1 lb of water from 63°F to 64°F
Slide 3
More About Heat
Heat is a microscopic form of energy transfer involving large numbers of particles
Energy exchange occurs due to individual interactions of the particles
No macroscopic displacements or forces involved
Heat flow is from a system at higher temperature to one at lower temperature
Flow of heat tends to equalize average microscopic kinetic energy of molecules
When 2 systems are in thermal equilibrium, they are at the same temperature and there is no net heat flow
Energy transferred by heat does not always mean there is a temperature change (see phase changes)
Slide 4
Heat Transfer Simulation
Simulation presented in class.
(ActivPhysics Online Exercise #8.6, copyright Addison Wesley publishing)
Slide 5
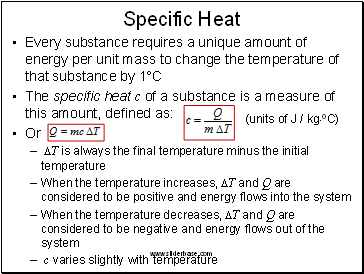
Specific Heat
Every substance requires a unique amount of energy per unit mass to change the temperature of that substance by 1°C
The specific heat c of a substance is a measure of this amount, defined as:
Or
DT is always the final temperature minus the initial temperature
When the temperature increases, DT and Q are considered to be positive and energy flows into the system
When the temperature decreases, DT and Q are considered to be negative and energy flows out of the system
Contents
- Heat and Internal Energy
- Units of Heat
- More About Heat
- Heat Transfer Simulation
- Specific Heat
- Consequences of Different Specific Heats
- Calorimetry
- Phase Transitions
- Evaporation and Condensation
- Conduction
- Home Insulation
- Convection
- Thermal Radiation
- Applications of Thermal Radiation
- Resisting Energy Transfer
- Global Warming
Last added presentations
- Mechanics Lecture
- Sound
- History of Modern Astronomy
- Upcoming Classes
- Static and Kinetic Friction
- Newton’s laws of motion
- Newton's laws of motion