Thermal EnergyPage
3
3
Common phase transitions are
Solid liquid (melting)
Liquid gas (boiling)
Phase transitions involve a change in the internal energy, but no change in temperature
Kinetic energy of molecules (which is related to temperature) is not changing, but their potential energy changes as work is done to change their positions
Energy required to change the phase of a given mass m of a pure substance is:
L = latent heat – depends on substance and nature of phase transition
+ (–) sign used if energy is added (removed)
Slide 12
Phase Transitions
All phase changes can go in either direction
Heat flowing into a substance can cause melting (solid to liquid) or boiling (liquid to gas)
Heat flowing out of a substance can cause freezing (liquid to solid) or condensation (gas to liquid)
Latent heat of fusion Lf is used for melting or freezing
Latent heat of vaporization Lv is used for boiling or condensing (somewhat larger for lower pressures)
Table 11.2 gives the latent heats for various substances
Large Lf of water is partly why spraying fruit trees with water can protect the buds from freezing
In process of freezing, water gives up a large amount of energy and keeps bud temperature from going below 0°C
Slide 13
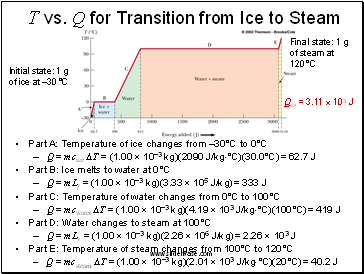
T vs. Q for Transition from Ice to Steam
Part A: Temperature of ice changes from –30°C to 0°C
Q = mcice DT = (1.00 10–3 kg)(2090 J/kg°C)(30.0°C) = 62.7 J
Part B: Ice melts to water at 0°C
Q = mLf = (1.00 10–3 kg)(3.33 105 J/kg) = 333 J
Part C: Temperature of water changes from 0°C to 100°C
Q = mcwater DT = (1.00 10–3 kg)(4.19 103 J/kg°C)(100°C) = 419 J
Part D: Water changes to steam at 100°C
Q = mLv = (1.00 10–3 kg)(2.26 106 J/kg) = 2.26 103 J
Part E: Temperature of steam changes from 100°C to 120°C
Q = mcsteam DT = (1.00 10–3 kg)(2.01 103 J/kg°C)(20°C) = 40.2 J
Initial state: 1 g of ice at –30°C
Final state: 1 g of steam at 120°C
Qtot = 3.11 103 J
Slide 14
Evaporation and Condensation
The previous example shows why a burn caused by 100°C steam is much more severe than a burn caused by 100°C water
Steam releases large amount of energy through heat as it condenses to form water on the skin
Much more energy is transferred to the skin than would be the case for same amount of water at 100°C
Evaporation is similar to boiling
Molecular bonds are being broken by the most energetic molecules
Average kinetic energy is lowered as a result, which is why evaporation is a cooling process
Approximately the same latent heat of vaporization applies
Contents
- Heat and Internal Energy
- Units of Heat
- More About Heat
- Heat Transfer Simulation
- Specific Heat
- Consequences of Different Specific Heats
- Calorimetry
- Phase Transitions
- Evaporation and Condensation
- Conduction
- Home Insulation
- Convection
- Thermal Radiation
- Applications of Thermal Radiation
- Resisting Energy Transfer
- Global Warming
Last added presentations
- Radioactivity and Nuclear Reactions
- Newton’s law of universal gravitation
- Thermal Energy
- Sound
- Radiation
- Simulation at NASA for the Space Radiation Effort
- Resource Acquisition and Transport in Vascular Plants