Structure of the AtomPage
1
1
Slide 1
4.1 The Atomic Models of Thomson and Rutherford
4.2 Rutherford Scattering
4.3 The Classic Atomic Model
4.4 The Bohr Model of the Hydrogen Atom
4.5 Successes and Failures of the Bohr Model
4.6 Characteristic X-Ray Spectra and Atomic Number
4.7 Atomic Excitation by Electrons
CHAPTER 4 Structure of the Atom
In the present first part of the paper the mechanism of the binding of electrons by a positive nucleus is discussed in relation to Planckís theory. It will be shown that it is possible from the point of view taken to account in a simple way for the law of the line spectrum of hydrogen.
- Niels Bohr, 1913
Slide 2
Structure of the Atom
Pieces of evidence that scientists had in 1900 to indicate that the atom was not a fundamental unit:
There seemed to be too many kinds of atoms, each belonging to a distinct chemical element.
Atoms and electromagnetic phenomena were intimately related.
The problem of valence. Certain elements combine with some elements but not with others, a characteristic that hinted at an internal atomic structure.
The discoveries of radioactivity, of x rays, and of the electron.
Slide 3
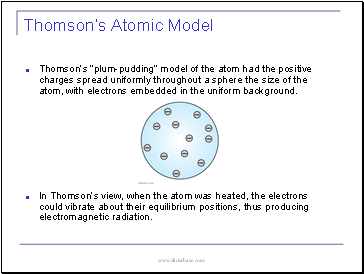
Thomsonís Atomic Model
Thomsonís ďplum-puddingĒ model of the atom had the positive charges spread uniformly throughout a sphere the size of the atom, with electrons embedded in the uniform background.
In Thomsonís view, when the atom was heated, the electrons could vibrate about their equilibrium positions, thus producing electromagnetic radiation.
Slide 4
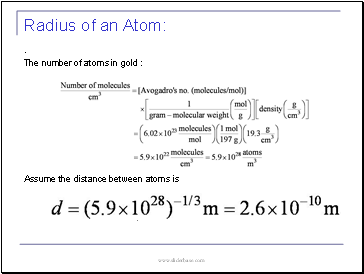
Radius of an Atom
.
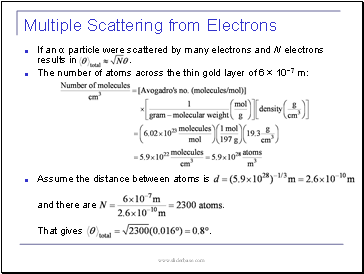
The number of atoms in gold :
Assume the distance between atoms is
.
.
:
Slide 5
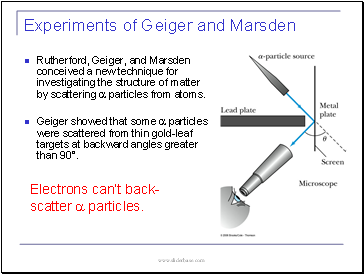
Experiments of Geiger and Marsden
Rutherford, Geiger, and Marsden conceived a new technique for investigating the structure of matter by scattering a particles from atoms.
Geiger showed that some a particles were scattered from thin gold-leaf targets at backward angles greater than 90į.
Electrons canít back-scatter a particles.
Slide 6
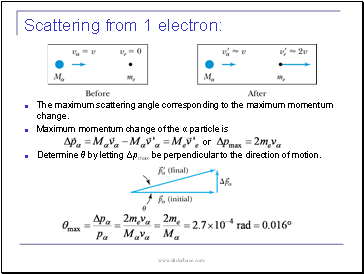
Scattering from 1 electron:
The maximum scattering angle corresponding to the maximum momentum change.
Maximum momentum change of the α particle is
or
Determine θ by letting Δpmax be perpendicular to the direction of motion.
Slide 7
Multiple Scattering from Electrons
Contents
- Structure of the Atom
- Thomsonís Atomic Model
- Radius of an Atom
- Experiments of Geiger and Marsden
- Scattering from 1 electron:
- Multiple Scattering from Electrons
- Rutherfordís Atomic Model
- Rutherford Scattering
- The Classical Atomic Model
- The Bohr Model of the Hydrogen Atom
- Atomic Excitation by Electrons
Last added presentations
- Practical Applications of Solar Energy
- Sensory and Motor Mechanisms
- Gravitation
- Resource Acquisition and Transport in Vascular Plants
- Magnetic field uses sound waves to ignite sun's ring of fire
- Health Physics
- Newtonís Laws of Motion