The Atom Atomic Number and Mass Number IsotopesPage
1
1
Slide 1
Chapter 2 Atoms and Elements
The Atom
Atomic Number and Mass Number
Isotopes
Slide 2
Atomic Theory
Atoms are building blocks of elements
Similar atoms in each element
Different from atoms of other elements
Two or more different atoms bond in simple ratios to form compounds
Slide 3
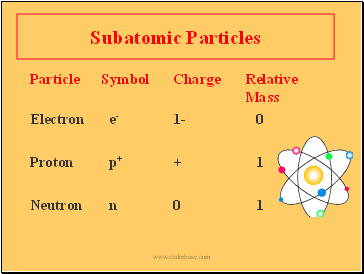
Subatomic Particles
Particle Symbol Charge Relative
Mass
Electron e- 1- 0
Proton p+ + 1
Neutron n 0 1
Slide 4
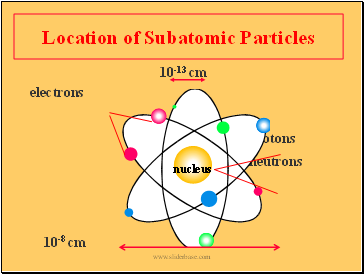
Location of Subatomic Particles
10-13 cm
electrons
protons
neutrons
10-8 cm
nucleus
Slide 5
Atomic Number
Counts the number
of
protons
in an atom
Slide 6
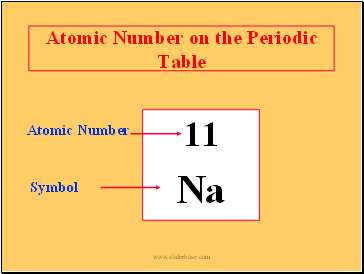
Atomic Number on the Periodic Table
11
Na
Atomic Number
Symbol
Slide 7
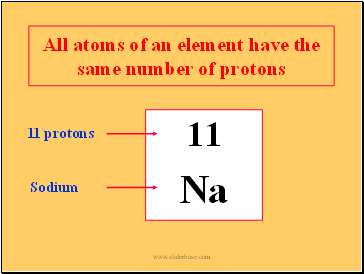
All atoms of an element have the same number of protons
11
Na
11 protons
Sodium
Slide 8
Learning Check
State the number of protons for atoms of each of the following:
A. Nitrogen
1) 5 protons 2) 7 protons 3) 14 protons
B. Sulfur
1) 32 protons 2) 16 protons 3) 6 protons
C. Barium
1) 137 protons 2) 81 protons 3) 56 protons
Slide 9
Solution
State the number of protons for atoms of each of the following:
A. Nitrogen
2) 7 protons
B. Sulfur
2) 16 protons
C. Barium
3) 56 protons
Slide 10
Mass Number
Counts the number
of
protons and neutrons
in an atom
Slide 11
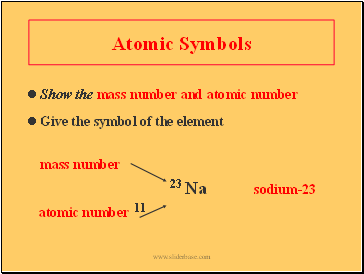
Atomic Symbols
Show the mass number and atomic number
Give the symbol of the element
mass number
23 Na sodium-23
atomic number 11
Slide 12
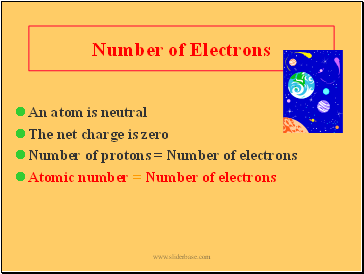
Number of Electrons
An atom is neutral
The net charge is zero
Number of protons = Number of electrons
Atomic number = Number of electrons
Slide 13
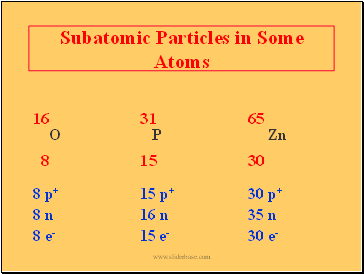
Subatomic Particles in Some Atoms
1 2
Contents
- Atomic Theory
- Subatomic Particles
- Location of Subatomic Particles
- Atomic Number
- Atomic Number on the Periodic Table
- All atoms of an element have the same number of protons
- Learning Check
- Solution
- Mass Number
- Atomic Symbols
- Number of Electrons
- Subatomic Particles in Some Atoms
- Isotopes
- Atomic Mass on the Periodic Table
- Atomic Mass
Last added presentations
- Motion
- Upcoming Classes
- Friction
- Space Radiation
- Magnetic field uses sound waves to ignite sun's ring of fire
- Solar Energy
- History of Modern Astronomy