Metals and Acids expsPage
3
3
magnesium is more reactive than iron
and
lead + copper (II) sulphate lead (II) sulphate + copper
lead is more reactive than copper
Slide 30
DISPLACEMENT REACTIONS
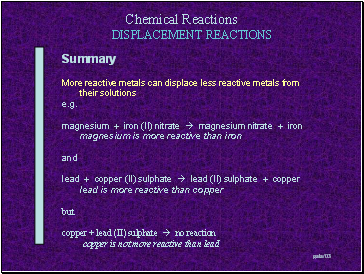
Summary
More reactive metals can displace less reactive metals from their solutions
e.g.
magnesium + iron (II) nitrate magnesium nitrate + iron
magnesium is more reactive than iron
and
lead + copper (II) sulphate lead (II) sulphate + copper
lead is more reactive than copper
but
copper + lead (II) sulphate
Slide 31
DISPLACEMENT REACTIONS
Summary
More reactive metals can displace less reactive metals from their solutions
e.g.
magnesium + iron (II) nitrate magnesium nitrate + iron
magnesium is more reactive than iron
and
lead + copper (II) sulphate lead (II) sulphate + copper
lead is more reactive than copper
but
copper + lead (II) sulphate no reaction
Slide 32
DISPLACEMENT REACTIONS
Summary
More reactive metals can displace less reactive metals from their solutions
e.g.
magnesium + iron (II) nitrate magnesium nitrate + iron
magnesium is more reactive than iron
and
lead + copper (II) sulphate lead (II) sulphate + copper
lead is more reactive than copper
but
copper + lead (II) sulphate no reaction
copper is not more reactive than lead
Contents
Last added presentations
- Upcoming Classes
- Newton's laws of motion
- Buoyancy
- Health Physics
- Gravitation
- Sound
- The Effects of Radiation on Living Things